NANO230 North Seattle Community College Homework #3
advertisement

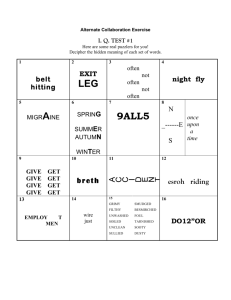
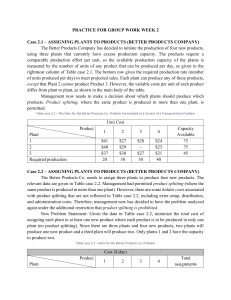
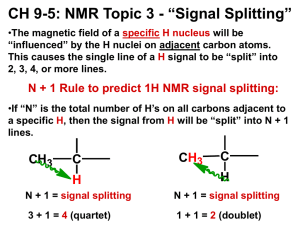
NANO230 North Seattle Community College Homework #3 Due:_______________________ Solar-Driven Water Splitting Read through the Lab Manual: Discovering New Materials for Solar-Driven Water Splitting. Answer the following on a separate page. 1. What is the chemical equation that describes water splitting? What can the products of this reaction be used for? In what ways is water splitting advantageous compared to photovoltaics? 2. What are the chemical equations for the half reactions? Indicate which half reaction is the oxidation of water, and which half reaction is the reduction of H+ to H2. Indicate which half reaction occurs at the carbon counter electrode, and which half reaction occurs at the metal oxide spot. 3. How much potential (voltage) is required to drive the water splitting reaction? What wavelength range (in nm) and colors of the solar spectrum has the energy necessary to split water? [Hint: consider the potential in eV and convert to nm) 4. Refer to Figure 5 in the Lab Manual, which shows band gaps and band energies. Is silicon a good material for water splitting? Give two specific reasons to support your argument.