METABOLISM OVERVIEW
advertisement

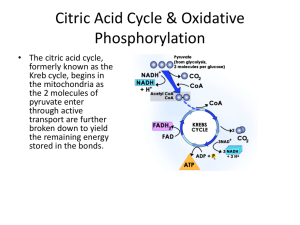
METABOLISM OVERVIEW METABOLISM • The sum of all reactions occurring in an organism, includes: • catabolism, which are the reactions involved in the breakdown of biomolecules. • anabolism, which are the reactions involved in the synthesis of biomolecules. • A metabolic pathway is a sequence of reactions used to produce one product or accomplish one process. CATABOLISM OF FOOD • The catabolism of food is a three stage process. • Stage I: Large, complex molecules are digested into simpler ones, using hydrolysis reactions. CATABOLISM OF FOOD (continued) • Stage II: Small molecules are broken down into simpler units, usually two carbon portion of acetyl CoA. • Stage III: The “common catabolic pathway” extracts energy to produce ATP. CATABOLISM OF FOOD (continued) CARBOHYDRATE CATABOLISM CARBOHYDRATE METABOLISM (DIGESTION) • The main reaction of carbohydrate digestion to monosaccharides is hydrolysis: Glucose Metabolism Overview Glycogen Glycogenesis High glucose present, β cells in pancreas realease insulin Ribose RNA Polysaccharide Energy Storage Glycogenolysis Muscles need energy or fear, anger conditions or absence of glucose Glucose Gluconeogensis During starvation breakdown of proteins from muscle cells Glycolysis 10 step process Pyruvate Energy GLYCOLYSIS • Glucose (C6) is catabolically oxidized through a many step process to pyruvate (C3). • In addition to the two molecules of pyruvate, two molecules of ATP, two molecules of NADH, four molecules of H+, and two molecules of H2O are produced. • Glycolysis occurs in the cellular cytoplasm. • Net reaction for glycolysis: GLYCOLYSIS (continued) GLYCOLYSIS (continued) GLYCOLYSIS – REGULATION (continued) THE FATES OF PYRUVATE (continued) PYRUVATE OXIDATION TO ACETYL CoA • Pyruvate oxidation to acetyl CoA occurs in the mitochondria. • Most of the acetyl CoA will be completely oxidized to CO2 in the citric acid cycle. • Some acetyl CoA will serve as starting material for fatty acid biosynthesis. • NAD+ is regenerated when NADH transfers its electrons to O2 in the electron transport chain. PYRUVATE REDUCTION TO LACTATE • Pyruvate reduction to lactate occurs in cells after strenuous or long-term muscle activity because the cellular supply of oxygen is not adequate for the reoxidation of NADH to NAD+. • Under anaerobic conditions, animals and some microorganism can obtain limited energy through lactate fermentation. PYRUVATE REDUCTION TO ETHANOL • Under anaerobic conditions, some microorganisms can obtain limited energy through glycolysis and the two step conversion of pyruvate to ethanol. • Overall equation: • Step-wise equations: LIPID CATABOLISM LIPID METABOLISM – BLOOD LIPIDS • During digestion, lipids are hydrolyzed to glycerol, fatty acids, and monoglycerides. • For transport in the lymph and blood, the cells of the small intestines produce lipoprotein aggregates called chylomicrons. BLOOD LIPID CLASSIFICATION • Lipids are less dense than proteins. • The classification of blood lipids is based on density. • The higher the lipid concentration of a lipoprotein aggregate, the lower the density. VLDL = very low density lipoprotein LDL = low density lipoprotein HDL = high density lipoprotein SCHEMATIC MODEL OF LDL GLYCEROL METABOLISM • Glycerol can be converted to dihydroxyacetone phosphate, an intermediate of glycolysis. • Glycerol can be converted to pyruvate and contribute to cellular energy production. • Pyruvate can be converted to glucose through gluconeogenesis. FATTY ACID OXIDATION Activation Step: • Before fatty acids can be catabolized, they must be activated by conversion to fatty acyl CoA. • The conversion to fatty acyl CoA is catalyzed by acyl CoA synthetase. FATTY ACID OXIDATION (continued) FATTY ACID OXIDATION (continued) O O CH3(CH2)14-C-CH2-C-S-CoA FATTY ACID OXIDATION (continued) • Stearic acid: • makes eight passes through -oxidation sequence. • produces nine molecules of acetyl CoA. • produces eight molecules FADH2. • produces eight molecules of NADH. FATTY ACID OXIDATION (continued) • In the last spiral, the four-carbon chain of butyryl CoA passed through the -oxidation sequence, and produces: • one molecule FADH2, • one molecule NADH, • and two molecules of acetyl CoA. KEY NUMBERS FOR ATP CALCULATIONS • Determine the number of acetyl CoA molecules: number of fatty acid carbons acetyl CoA 2 • Determine the number of trips through the fatty acid spiral (one less than the number of acetyl CoA molecules): • Multipliers: • every acetyl CoA = 10 ATP molecules • every NADH = 2.5 ATP molecules • every FADH2 = 1.5 ATP molecules KEY NUMBERS FOR ATP CALCULATIONS (continued) • Example for 10-carbon fatty acid (five acetyl CoA molecules, four trips through fatty acid spiral): (5 acetyl CoA) × 10 (4 NADH + H+) × 2.5 (4 FADH2) × 1.5 Activation step Total ATP = 50 ATP = 10 ATP = 6 ATP = −2 ATP = 64 ATP ENERGY FROM FATTY ACIDS (continued) • The amount of energy produced from stearic acid (C18): KEY COMPONENTS IN CATABOLISM ATP – THE PRIMARY ENERGY CARRIER (continued) • ATP hydrolysis releases a great amount of free energy: • ATP is a high-energy compound (liberates a great amount of free energy on hydrolysis). ATP – THE PRIMARY ENERGY CARRIER (continued) ATP-ADP CYCLE • Supplies cellular energy ATP FORMATION • ATP formation occurs on the inner membrane of mitochondria (cellular organelle where reactions of the common catabolic pathway occur). IMPORTANT COENZYMES – COENZYME A • Coenzyme A is a central compound in metabolism, it is part of acetyl CoA. • It is derived from the B vitamin pantothenic acid. • It contains a reactive sulfhydryl group (CoA-SH). • It forms a thioester bond with an acetyl group or other acyl groups. • Acetyl CoA is the primary fuel for citric acid cycle. IMPORTANT COENZYMES – NAD+ • Nicotinamide adenine dinucleotide (NAD+) is a derivative of ADP and the vitamin nicotinamide. NAD+ • NAD+ acts as an electron acceptor. NAD+ (continued) • NAD+ is important in the oxidation of many biomolecules. IMPORTANT COENZYMES – FAD • Flavin adenine dinucleotide (FAD) is derived from ADP and the vitamin riboflavin. FAD • FAD acts as an electron acceptor. FAD (continued) • FAD is often involved in the oxidation of –CH2–CH2– to –CH=CH– bonds. CITRIC ACID CYCLE (KREBS CYCLE) THE CITRIC ACID CYCLE • The citric acid cycle: • has other names, including: • the tricarboxylic acid cycle. • the Krebs cycle. • is the principle process for generating the reduced coenzymes NADH and FADH2. • is the source of intermediates for biosynthesis. • occurs within the matrix of the mitochondrion. • includes eight reactions. THE CITRIC ACID CYCLE (continued) • Pyruvate oxidized to acetyl CoA can enter the citric acid cycle where it will be further oxidized to two molecules of CO2, producing one molecule of GTP and the reduced forms of three molecules of NAD+ (NADH) and one molecule of FAD (FADH2) which can then enter the electron transport chain to produce ATP. • The overall reaction is: THE CITRIC ACID CYCLE (continued) THE CITRIC ACID CYCLE (continued) THE CITRIC ACID CYCLE (continued) CITRIC ACID CYCLE – REGULATION (continued) •The rate of citric acid cycle is reduced when cellular ATP levels are high. •The rate of citric acid cycle is increased when ATP supplies are low and ADP levels are high. THE ELECTRON TRANSPORT CHAIN • NADH and FADH2 are produced by the citric acid cycle. • They enter the electron transport chain where they can be used to supply hydrogen ions and electrons to reduce oxygen to water. • Net equation: 4H+ + 4e− + O2 → 2H2O • The electron transport chain occurs in a series of reactions. THE ELECTRON TRANSPORT CHAIN (continued) • The electron transport chain is found in the inner membrane of the mitochondria and involves iron-containing enzymes (cytochromes). THE ELECTRON TRANSPORT CHAIN (continued) • As electrons are transported along the electron transport chain, a significant amount of free energy is released (52.6 kcal/mol). • Some free energy is conserved in oxidative phosphorylation (production of ATP from ADP and Pi). OXIDATIVE PHOSPHORYLATION: CHEMIOSMOTIC HYPOTHESIS • The chemiosmotic hypothesis states that the electron transport chain pumps H+ across the inner mitochondrial membrane, H+ then flows back across the membrane, causing the formation of ATP by F1-ATPase. • Oxidative phosphorylation conserves approximately 34% of the energy released from the electron transport chain for each mole of NADH. • Oxidative phosphorylation conserves approximately 25% of the energy released from the electron transport chain for each mole of FADH2. OXIDATIVE PHOSPHORYLATION: CHEMIOSMOTIC HYPOTHESIS OXIDATIVE PHOSPHORYLATION FROM ELECTRON TRANSPORT • The conversion of NADH to NAD+ generates 2.5 ATP from ADP during oxidative phosphorylation. • The conversion of FADH2 to FAD generates 1.5 ATP from ADP during oxidative phosphorylation. • The energy yield for the entire catabolic pathway (citric acid cycle, electron transport chain, and oxidative phosphorylation combined):