Study Guide and Problems – Exam 2 and 3
advertisement

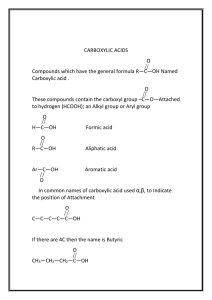
CHEM 131 – Spring 2011 Study Guide and Problems – Exam 2 and 3 Nomenclature: The root is always the same no matter what ending you use for the different class of compounds Butane CH3CH2CH2CH3 Cyclobutane H2C CH2 H2C CH2 1-Butanol CH3CH2CH2CH2-OH OH 1-Butanethiol CH3CH2CH2CH2-SH Dibutyl ether CH3CH2CH2CH2-O-CH2CH2CH2CH3 1-Butene CH3CH2CH=CH2 1-Butyne H3C SH CH2 C O CH C Butanal CH3 CH2 CH2 2-Butanone C H C CH3 Butanoic acid CH2 CH2 C OH CH2 Methyl Butanoate O C CH2 CH2 O O O O CH3 O Carboxylic acid that is deprotonated (missing a H+) O CH2 O OH Butanoate CH3 H Carbonyl carbon with 1 OH and 1 alkyl group O CH3 O Carbonyl carbon with 2 alkyl groups O CH3 CH2 CH Carbonyl carbon with 1 H and 1 alkyl group O C O CH3 O 1-Butamine CH3 CH2 CH3 CH2 CH2 CH2 NH2 NH2 N-methyl-1-butamine CH2 CH2 NH CH3 N H N,N-dimethyl-1-butamine CH3 1-Butammonium ion CH3 CH2 CH2 CH2 N CH3 CH2 CH2 NH3+ CH2 Butanamide CH2 CH2 N-Methylbutanamide C CH2 CH2 C NH3 NH2 NH2 O O CH3 Protonated amine O O CH3 N 2 NH CH3 N H CHEM 131 – Spring 2011 O N,N-Dimethylbutanamide O CH3 As a functional group: Butyl CH2 CH2 C N CH3 N 2 R-CH2CH2CH2CH3 R Di-, tri-, tetra-, penta-, hexa- etc. is only used if the same functional group appears several times on the same molecule. For example: 2 methyl groups would be dimethyl or 4 methyl groups would be tetramethyl Common Name Items: Acetic acid Toluene O CH3 OH Acetate (acetic acid as functional group) Phenol O OH R O Benzene Aniline NH2 Phenyl (Benzene as functional group) Benzaldehyde O R H O Benzoic acid OH To determine if an alcohol is a primary, secondary or tertiary, you need to look at the carbon atom to which the hydroxyl group is attached to. Depending on how many other carbon atoms are bound to the hydroxyl carbon determines if it is primary, secondary or tertiary. Fill in the circles with C or H depending on your structure and then look at the table. Type of alcohol 1 (primary) 2 (secondary) 3 (tertiary) # of carbons 1 2 3 # of hydrogens 2 1 0 OH C CHEM 131 – Spring 2011 If you are trying to determine if an amine is a primary, secondary or tertiary, you now replace the C with the N and the OH with lone pair of electrons on the picture for alcohols. Depending on how many other carbon atoms are bound to the nitrogen determines if it is primary, secondary or tertiary. Fill in the circles with C or H depending on your structure and then look at the table. Type of alcohol 1 (primary) 2 (secondary) 3 (tertiary) # of carbons 1 2 3 # of hydrogens 2 1 0 N Some of the reactions from the past Reduction or Hydrogenation: Addition of hydrogen across double bonds (across C=C or C=O double bonds) H R' R Pt R H H C C H H H2 H R' O H2 HO This second reaction is part of your oxidation/reduction scheme of alcohols/aldehydes/ketones/carboxylic acids. H Pt Oxidation (sometimes called elimination): Loss of hydrogen to form a C=C or C=O bond R H H C C H H R' (ox) H R' R H - H2 HO O H This second reaction is part of your oxidation/reduction scheme of alcohols/aldehydes/ketones/carboxylic acids. (ox) - H2 Strictly speaking, the formation of a disulfide bond is considered an oxidation, since hydrogen is removed. (ox) SH - H2 + HS S S Hydration: Addition of water across a C=C double bond to make alcohols H R' + H2O H H H2SO4 H H OH C C H H R' Remember Markovnikov’s rule: the rich get richer – the hydrogen will add to the carbon that already has more carbons CHEM 131 – Spring 2011 Addition of hydrogen halide: Addition of acids such as HF, HCl, HBr or HI. H R' H2SO4 + HBr H H H H Br C C H H R' Again this reaction follows Markovnikov’s rule: the rich get richer – the hydrogen will add to the carbon that already has more carbons Dehydration: Loss of water from an alcohol to make a C=C double bond or an ether H OH C C H H CH2 OH H R' R H H2SO4 R R R' 180C + H O CH2 H2SO4 R R 140C At higher temperature the dehydration is the preferred reaction, but at lower temperature the + H2O formation of ethers is preferred. CH2 O CH2 R Oxidation/Reduction of Alcohols, Aldehydes, Ketones, Carboxylic acid The key to remember for oxidation reactions of alcohols and aldehyde is the following table: 1° Alcohol Aldehyde 2° Alcohol Ketone 3° Alcohol No reaction Carboxylic acid (the last step requires the presence of water) The reaction schemes below show the same information on generic alcohols. In each case 2 protons and 2 electrons get removed. If there are no H on the carbon that is involved in the oxidation, then no oxidation can occur. O H-OH O H R C O H H (ox) (ox) R H Aldehyde 2 H+ + 2 e- R 2 H+ + 2 e- 1Alcohol O H R C O H R' Ketone (ox) R R' 2 H+ + 2 e- 2Alcohol R" R C O R' 3Alcohol H (ox) No Reaction OH Carboxylic Acid CHEM 131 – Spring 2011 For reductions, you simply march backwards on the reaction schemes. Here we are adding hydrogens back in. O O H H2, Pt H2, Pt R (red) OH R H-OH Carboxylic Acid R (red) H C O H H Aldehyde 1Alcohol H O H2, Pt Ketone R R' (red) R C O H R' 2Alcohol Review of Reactions involving amines, carboxylic acids, esters, thioesters and amides Amines as weak base (amines accept protons to form ammonium ions): Since amines are weak bases, they easily accept a proton form water and acids and become protonated, forming an ammonium ion: H H N CH2CH3 + H2O H H N CH2CH3 + OH H Likewise, amines will react with strong acids to form the ammonium ion. The chlorine acts as a counter ion in the salt. H H N H CH2CH3 + HCl H N H CH2CH3 Cl CHEM 131 – Spring 2011 Carboxylic acids as weak acids (they can donate a proton): Carboxylic acids are weak acids and therefore will dissociate in water O O + H3O + H2O H R R O This gives a carboxylate ion, which is the conjugate base of the carboxylic acid. O And carboxylic acids will react with strong bases. This leads to a carboxylate salt. In this case a sodium carboxylate. Water is the side + H2O product. O O H R + NaOH O Na R O Esters, Thioesters and Amides: The secret of esters, thioesters and amides: they are made up of a carboxylic acid combined with either alcohol, thiol or amine, respectively. As you can see they are virtually identical. Here a generic carboxylic acid, R-COOH, is shown along with ethanol, ethanethiol, and ethanamine. O Thioester O Ester R O O S CH2CH3 R O R OH Amide CH2CH3 CH2CH3 R O + HO-CH2CH3 N H O R OH + HS-CH2CH3 R OH + H2N-CH2CH3 Ester, Thioester and Amide Formation: The formation of these esters, thioesters and amides are very similar also. They can be formed by either using carboxylic acid combined with acid and heat, or two carboxylic acid derivatives, which are more reactive and lead to higher yield than carboxylic acids. These consist of a carboxylic acid chloride or a carboxylic acid anhydride. In each of these reactions the side products are slightly different. An example is shown for the formation of ester via carboxylic acid and alcohol coupling using acid and heat: O O + R OH + HO-CH2CH3 H , heat CH2CH3 R O + H2O CHEM 131 – Spring 2011 Below is an example of thioester formation. O O + HS-CH2CH3 R H+, heat CH2CH3 OH R + H2O S As long as there is a proton on the amine, amide formation is possible. O O H+, heat + HN-CH2CH3 R OH R H CH2CH3 + H2O CH2CH3 + H2O N H O O + H , heat + HN-CH2CH3 R OH R CH3 CH3 O R N OH + CH3-N-CH2CH3 H+, heat No Reaction CH3 Hydrolysis of esters, thioesters and amides: When we think about cleaving the ester, thioester or amide bond, we can think of it as getting the original components back. This reaction is your simple hydrolysis reaction where “water” adds across the bond to be broken either directly or indirectly, depending of the reaction conditions. Ester Carboxylic acid + alcohol Thioester Carboxylic acid + thiol Amide Carboxylic acid + amine This can be done either under acidic or basic conditions. And that is where most of the confusion stems from. The only thing to consider is, how does the reaction conditions affect the products that are being formed. We already established that a carboxylic acid is a weak acid and that it reacts with strong bases. On the other hand, it is not affected by any acid present. Amines are weak bases and will react with strong acids, but are unaffected by bases. Alcohols and thiols are unaffected by both acids and bases. How do we translate this to reality? Simple – first, write down the two products you would get if everything is neutral (ester carboxylic acid + alcohol). Secondly, think of the effect the reaction conditions have on the products. Carboxylic acid will react with base; and an amine will react with acid. So if we look at the hydrolysis of esters, we see that under acidic conditions, neither product is affected by the acid. On the other hand, when base is used, the alcohol remains unchanged, but the carboxylic acid undergoes acid-base chemistry and is deprotonated forming the carboxylate with sodium acting as counterion. CHEM 131 – Spring 2011 O Acidic hydrolysis O + CH2CH3 R Basic hydrolysis + H2O H , heat + HO-CH2CH3 R O O O CH2CH3 R OH + HO-CH2CH3 + NaOH R O O Na Thioesters behave the same identical way as alcohols, only a thiol will result as one of the products. Now, when we look at amides, both the carboxylic acid and the resulting amine are vulnerable to acid or base conditions. Carboxylic acid will react with the NaOH, whereas the amine will react with HCl. See acid/base behavior of those two compounds shown above. Again the chlorine and sodium act as counterions in the resulting salt. O O Acidic hydrolysis CH2CH3 R + H2O + R N H H N CH2CH3 OH H O O Basic hydrolysis CH2CH3 R H H+, heat N H + NaOH R O Na + HN-CH2CH3 H Cl