CHAPTER 15 The 3-D Shape of Molecules General, Organic, & Biological Chemistry
advertisement

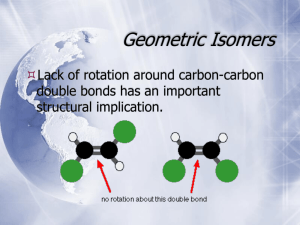
CHAPTER 15 The 3-D Shape of Molecules General, Organic, & Biological Chemistry Janice Gorzynski Smith 1 CHAPTER 15: The 3-D Shape of Molecules Learning Objectives: Understand the difference between Chiral and Achiral molecules Identify chiral centers Draw the enantiomers & diastereomers of a chiral molecule (using dashes & wedges to show geometry) Draw fischer projections of chiral molecules Explain the relevance of chiral centers in Biochemistry 2 Smith. General Organic & Biological Chemistry 2nd Ed. ISOMERS Same chemical formula Stereoisomers Constitutional Isomers Spatial Isomers Structural Isomers Different connectivity between atoms Different spatial arrangement of atoms but with the same connectivity Enantiomers Diastereomers Mirror images of a chiral molecule that are not superimposable All stereoisomers that are not mirror images of one another Cis/Trans Isomers Conformational Isomers Rotation around C-C single bonds R R R R 3 Stereoisomers Chiral & Achiral •Left hands and right hands are mirror images of each other. •A molecule that is not superimposable on its mirror image is chiral. 4 Smith. General Organic & Biological Chemistry 2nd Ed. Stereoisomers Chiral & Achiral •Two socks from a pair are mirror images that are superimposable. •A molecule that is superimposable on its mirror image is achiral. Smith. General Organic & Biological Chemistry 2nd Ed. 5 Stereoisomers Chiral & Achiral To test whether a molecule is chiral or achiral: 1. Draw the molecule in 3 dimensions. 2. Draw its mirror image. 3. Try to align all bonds and atoms. •To superimpose a molecule and its mirror image you can perform any rotation but you cannot break bonds. 6 Smith. General Organic & Biological Chemistry 2nd Ed. Stereoisomers Chiral & Achiral For CH2BrCl: Rotate the molecule to align bonds: ACHIRAL 7 Smith. General Organic & Biological Chemistry 2nd Ed. Stereoisomers Chiral & Achiral For CHBrClF: CHIRAL 8 Smith. General Organic & Biological Chemistry 2nd Ed. Stereoisomers Chiral & Achiral Identify the chiral centers in… cholesterol glucose cysteine 9 Chiral Molecules Enantiomers Many drugs are chiral, and often they must interact with a chiral receptor to be effective. Thalidomide was an anti-nausea drug sold to pregnant in the 60’s as a mixture with its enantiomer. It was found that the enantiomer interacted with the body differently, and caused catastrophic birth defects. 10 Smith. General Organic & Biological Chemistry 2nd Ed. Chiral Molecules Enantiomers Ibuprofen is an active anti-inflammatory agent whose enantiomer is inactive, and it is sold as a racemic mixture. 11 Smith. General Organic & Biological Chemistry 2nd Ed. Chiral Molecules Enantiomers Two different enantiomers can interact differently with smell receptors in the brain and have different perceived odors. 12 Smith. General Organic & Biological Chemistry 2nd Ed. Stereoisomers Multiple Chiral Centers Draw the Enantiomer of the amino acid Glutamate 13 Chiral Molecules Multiple Chiral Centers 14 Smith. General Organic & Biological Chemistry 2nd Ed. Stereoisomers chemwiki.ucdavis.edu Multiple Chiral Centers 15 Chiral Molecules Fischer Projections A Fischer Projection takes a 3D tetrahedral shape, and re-draws it: 16 Smith. General Organic & Biological Chemistry 2nd Ed. Chiral Molecules Fischer Projections We can draw the cross and Fischer projection for both enantiomers of 2-butanol: 17 Smith. General Organic & Biological Chemistry 2nd Ed. Chiral Molecules Fischer Projections Fischer Projections with multiple chiral centers: A&C; A&D; B&C; B&D are all Diastereomers Smith. General Organic & Biological Chemistry 2nd Ed. 18 Chiral Molecules Fischer Projections Draw a Fischer Projections of the carbohydrate erythrose’s stereoisomers (all 4). 19 Chiral Molecules Optical Properties •Two enantiomers have identical physical properties (melting pt., boiling pt., solubility, etc.) •They differ in how they interact with plane-polarized light. 20 Smith. General Organic & Biological Chemistry 2nd Ed. Chiral Molecules Optical Properties •An achiral compound does not change the direction of plane-polarized light and is optically inactive. •A chiral compound rotates the plane of polarized light through an angle α and is optically active. 21 Smith. General Organic & Biological Chemistry 2nd Ed. Chiral Molecules Optical Properties Assign Priority to the substituents bonded to the chiral carbon 1-highest, 4-lowest, to determine R or S rotation 1. The higher the atomic number of the immediate substituent atom, the higher the priority. For example, H– < C– < N– < O– < Cl–. 2. If two substituents have the same immediate substituent atom, evaluate atoms progressively further away from the chiral center until a difference is found. For example, CH3– < C2H5– < ClCH2– < BrCH2– < CH3O–. 3. If double or triple bonded groups are encountered as substituents, they are treated as an equivalent set of singlebonded atoms. For example, C2H5– < CH2=CH– < HC≡C– 22 https://www2.chemistry.msu.edu Chiral Molecules Optical Properties: R & S Enantiomers have opposite optical properties: ie, R,R vs S, S rotation of light One of the chiral centers of diastereomers will rotate light in the same way: ie, R,R vs R,S 23 chemwiki.ucdavis.edu Chiral Molecules Optical Properties: L & D L is “left handed” (equivalent to “S”) D is “right handed” (equivalent to “R”) 24