Ionic Compounds
advertisement

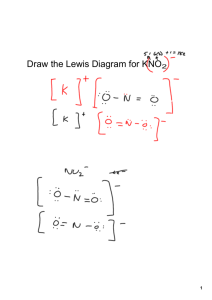
Ionic Compounds The joining of two atoms in a stable arrangement Elements gain, lose, or share electrons to reach the electron configuration of the noble gas closest to them in the periodic table Two types of bonding ◦ Ionic bonds: result from the transfer of electrons from one element to another ◦ Covalent bonds: result from the sharing of electrons between two atoms A pure substance that cannot be broken down into simpler substances by a chemical reaction Identified by a one- or two-letter symbol Arranged in the periodic table Its location on the periodic table indicates a lot about its chemical properties Can be a metal, nonmetal, or a metalloid Ionic bonds ◦ Form between a metal (left side of periodic table) and a nonmetal (right side of periodic table Covalent bonds ◦ Form when two nonmetals combine ◦ Form when a metalloid bonds to a nonmetal Positively charged ions Have fewer elections (e-) than protons sodium atom sodium ion Negatively charged ions Has more e- than protons ◦ chlorine atom chlorine ion A main group element is especially stable when it possesses an octet of e- in its outer shell octet = 8 valence e− the cation charge = the group number group 1A: M 1 valence e− M + + e− group 2A: M 2 valence e− M2+ + 2e− group 3A: M 3 valence e− M3+ + 3e− the anion charge = 8 – group number group 6A: group 7A: + X 6 valence e− 2e− + e− X 7 valence e− X 2− charge = 8 – 6 = 2 X − charge = 8 – 7 = 1 The sum of the charges in an ionic compound must be zero overall Ions will arrange to maximize anion-cation attractions and minimize anion-anion and cation-cation repulsions There must be two F- anions for each Ca+ cation to have an overall charge of zero HOW TO Write a Formula for an Ionic Compound Step [1] Identify which element is the cation and which is the anion. Step [2] Determine how many of each ion type is needed for an overall charge of zero. When the cation and anion have different charges, use the ion charges to determine the number of ions of each needed. Step [3] To write the formula, place the cation first and then the anion, and omit charges. Calcium Iodide ◦ Cation = calcium Charge 2+ ◦ Anion = iodine Charge - ◦ Need two iodine for every calcium for an overall charge of zero ◦ CaI2 “Criss-cross” rule Make magnitude of charge on one ion into subscript for other When doing this, make sure that subscripts are reduced to lowest whole number. Al3+ O2– Al2O3 Main group cations are named for the element from which they are formed. Na+ sodium K+ potassium Ca2+ calcium Mg2+ magnesium Systematic name ◦ Follow the name of the cation by a Roman numeral in parentheses to indicate its charge Fe2+ iron(II) Fe3+ iron(III) Common name ◦ Use suffic “-ous” for the cation with a smaller charge and suffix “-ic” for the cation with a higher charge Fe2+ Fe3+ ferrous ferric Anions are named by replacing the ending of the element name by the suffix “-ide.” Name the cation and then the anion Do not specify the charge on the ion Do not specify how many ions of each type are needed to balance charge Na+ + sodium Mg2+ + magnesium F− fluoride Cl− chloride NaF sodium fluoride MgCl2 magnesium chloride HOW TO Name an Ionic Compound Step [1] Determine the charge on the cation. Step [2] Name the cation and the anion If the cation could be multiple charges indicate the charge with roman numerals or with a –ous / -ic suffix. Step [3] Write the name of the cation first then the name of the anion K 2O ◦ Cation = K+ ◦ Anion = O2◦ Potassium Oxide LiBr SnO ◦ Cation = Li+ ◦ Anion = Br◦ Lithium Bromide ◦ Cation = Sn2+ ◦ Anion = O2◦ Tin (II) Oxide /Stannous Oxide HOW TO Derive the Formula from the Name of an Ionic Compound Step [1] Identify the cation and the anion. Step [2] Determine the charge on the cation and anion. Step [3] Balance the charges. Step [4] Write the formula with the cation first and use subscripts to communicate charge balance. Cobalt (II) Oxide/Cobaltous Oxide ◦ Cation = Co ◦ Anion = O2◦ CoO 2+ Iron(III) Fluoride/Ferric Flouride Beryllium Chloride ◦ Cation = Fe3+ ◦ Anion = F◦ FeF3 ◦ Cation = Be2+ ◦ Anion = Cl◦ BeCl2 Ionic compounds are crystalline solids with very high melting and boiling points When ionic compounds dissolve in water, they separate into cations and anions, increasing the conductivity of the solution + NaCl water solution A cation or anion that contains more than one atom The multiple atoms are held together with covalent bonds The molecule has an overall charge associated with it When a cation and anion of equal charge combine, only one of each ion is needed Na+ + NO2− NaNO2 zero overall charge Ba2+ + SO42− BaSO4 zero overall charge When a cation and anion of unequal charge combine, use the ionic charges to determine the relative number of each ion that is needed Mg2+ +2 charge means 2 OH− anions are needed. + OH− Mg(OH)2 −1 charge means 1 Mg2+ anion is needed. zero overall charge • • • The same rules are followed for naming standard ionic compounds: Name the cation and then the anion. Do not specify the charge on the ions. Do not specify how many ions of each type are needed to balance charge. •Use –ite suffix if 1 or less Oxygen atoms in anion •Use –ate suffix if more Oxygen atoms in anion •Use bi- prefix or write hydrogen if H part of anion NaHCO3 Al2(SO4)3 sodium bicarbonate aluminum sulfate