Fundamentals of Biochemistry Chapter 18 Third Edition
advertisement

Fundamentals of
Biochemistry
Third Edition
Donald Voet • Judith G. Voet •
Charlotte W. Pratt
Chapter 18
Electron transport and oxidative phosphorylation
Copyright © 2008 by John Wiley & Sons, Inc.
The story so far:
We have turned glucose and water into CO2:
C6H12O6 + 6 H2O 6 CO2 + 24 H+ + 24 e–
So why are aerobic (that is, O2requiring) conditions needed?
Clearly, this has something to do
with the 24 electrons that we can’t
leave on the electron carriers!
The mitochondrial electron-transport chain (ETC)
will allow the 24 electrons to reduce oxygen into water:
24 H+ + 24 e– + 6 O2 12 H2O
Moreover, NADH and FADH2 will be oxidized back to NAD+ and
FAD; this will occur at redox centers that occur on enzymes
located on or in the inner membrane of the mitochondrion.
Eukaryotic cells contain between 800 and 2000 mitochondria,
each about the size of a bacterium. The inner membrane contains
a lot of folds to increase surface area.
The outer membrane of the mitochondrion contains
porins, which are transport proteins that permit the free
diffusion of molecules less than 10 kD.
The intermembrane
space has the same
concentrations of ions and
metabolites as cytosol.
By contrast, the inner
membrane permits free
movement to O2, CO2 and
H2O only.
The inner face of
the inner membrane
contains the
greatest density of
proteins and other
non-lipid particles,
so this is where
much of the action
of electron transport
and oxidative
phosphorylation
takes place.
But why the high
density on the outer
face?
ATP must be transported out of the matrix into the
intermembrane space (and thus to the cytosol) and ADP
brought in via the ADP-ATP translocator
It’s clever in
that it knows
which hinge to
“open” based
on the
substrate it
binds
ATP must be transported out of the matrix into the
intermembrane space (and thus to the cytosol) and ADP
brought in via the ADP-ATP translocator
It’s clever in
that it knows
which hinge to
“open” based
on the
substrate it
binds
One point: swapping
an ATP4– for an
ADP3– leads to a
charge imbalance but
the proton pump will
take care of that
Getting electrons in and out
of the matrix is trickier: for
this, the membrane uses
“shuttle” systems, which use
a molecule whose reduced
and oxidized species can be
transported across the
membrane.
The glycerophosphate
shuttle is shown uses 3phosphoglycerol to
move NADH’s 2
electrons to FADH2 for
use in the ETC.
Energy of NADH reduction:
oxidation: NADH
NAD+ + H+ + 2 e–
reduction: ½ O2 + 2 H+ + 2 e–
net:
NADH + ½ O2 + H+
H2O
E°’ = + 0.315 V
E°’ = + 0.815 V
NAD+ + H2O ΔE°’ = + 1.130 V
Energy of NADH reduction:
oxidation: NADH
NAD+ + H+ + 2 e–
reduction: ½ O2 + 2 H+ + 2 e–
net:
NADH + ½ O2 + H+
H2O
E°’ = + 0.315 V
E°’ = + 0.815 V
NAD+ + H2O ΔE°’ = + 1.130 V
ΔG°’ = – n F ΔE°’ ≈ – 218 kJ/mol NADH
Energy of NADH reduction:
oxidation: NADH
NAD+ + H+ + 2 e–
reduction: ½ O2 + 2 H+ + 2 e–
net:
NADH + ½ O2 + H+
H2O
E°’ = + 0.315 V
E°’ = + 0.815 V
NAD+ + H2O ΔE°’ = + 1.130 V
ΔG°’ = – n F ΔE°’ ≈ – 218 kJ/mol NADH
Given that ATP stores 30.5 kJ/mol, and given that 2.5 ATP will
be generated per NADH, the efficiency of this process is about
35%, but can be raised to 70% by altering concentrations.
Where does the rest of the free energy go?
Oxidation of NADH and FADH2 is carried out by the
electron-transport chain (ETC)
There are four
protein complexes
containing redox
centers, and
electron carrier
molecules, such as
coenzyme Q
(CoQ), that move
electrons from
complex to
complex
Oxidation of NADH and FADH2 is carried out by the
electron-transport chain (ETC)
We know of these
complexes due to
inhibitors that
block the action of
each complex
(shown in red)
Oxidation of NADH and FADH2 is carried out by the
electron-transport chain (ETC)
Note that FADH2
enters into the ETC
at a later (lower
potential) point
than NADH; in
fact, FADH2
misses complex I
that generates an
ATP. Thus not as
much ATP will be
generated by
oxidizing FADH2
Most of the ETC complex inhibitors can form stable anions through
resonance, which allows them to prevent electrons from
transferring to oxygen for reduction
The table shows the
potentials of different
molecules in the ETC. Of
note are the redox center
molecules listed in the blue
boxes: many iron-sulfur
clusters, heme-based
pigments and copper
complexes.
Schematic cross-section of the inner mitochondrial
membrane, showing the ETC complexes and the
translocation of protons across the membrane
Complex II is also present, but not shown since it is not required for
NADH oxidation
Iron-sulfur clusters, attached to their
proteins via cysteine residues. These
clusters may undergo one-electron
oxidations and reductions.
Similar to FADH2,
both flavin
mononucleotide
(FMN) and CoQ
are one- or twoelectron carriers
In bacterial ETC complex I, the complex binds 2 [Fe-S] and 7
[4Fe-4S] clusters in the spatial arrangement shown; the electrons
physically move from one cluster to the next – a length of 95 Å
Complex I =
NADH-coenzyme
Q oxidoreductase
Weird fact: In mammals, 7 of the 45 subunits in
Complex I are coded in the mitochondrial DNA;
the remainder are coded in nuclear DNA, and so
must be imported! By contrast, Complex II is
entirely nuclear DNA-coded.
Weird fact: In mammals, 7 of the 45 subunits in
Complex I are coded in the mitochondrial DNA;
the remainder are coded in nuclear DNA, and so
must be imported! By contrast, Complex II is
entirely nuclear DNA-coded.
Other weird fact: The electrons move between iron-sulfur
clusters by quantum tunneling – a property of the wave
nature of matter
How do the protons then get
pumped into the intermembrane
space? Unlike Na+ or K+, there are
no transport proteins that allow
movement of protons.
How do the protons then get
pumped into the intermembrane
space? Unlike Na+ or K+, there are
no transport proteins that allow
movement of protons.
By the use of a “proton wire”, as
shown for bacteriorhodopsin, a
proton-pumping pigment. Retinal
gains free energy from absorbing
photons, and causes pKa changes
in the numerous ionizable residues
on the α-helices. A proton is
moved from one residue to the
next due to these pKa changes
until it reaches the other side of
the molecule.
This shift in retinal
begins the proton
wire cascade.
In chicken ETC
complex II, the
complex binds
succinate, FAD, CoQ
and 3 different ironsulfur clusters plus a
cytochrome
(electron-transport
heme-based proteins)
Complex II = succinatecoenzyme Q oxidoreductase
Keilin, D., “On cytochrome, a
respiratory pigment, common to
animals, yeast, and higher plants”,
(1925) Proc. R. Soc. Lond. B Biol.
Sci. 98:312–339.
All cytochromes contain heme rings with iron bound, which can
transfer electrons readily. The heme ring must be surrounded by
protein to prevent non-specific transfers of electrons.
Complex III has three
cytochromes and an ironsulfur cluster; passes
electrons from reduced CoQ
to cytochrome c
Complex III = coenzyme Qcytochrome c oxidoreductase
CoQ carries two electrons and
yields one electron to two
different cytochrome c molecules
via the Q cycle, which also is the
mechanism for complex III to
pump protons into the
intermembrane space.
CoQH2 + 2 cytochrome c1 (Fe3+) + 2 H+ (matrix)
CoQ + 2 cytochrome c1 (Fe2+) + 4 H+ (intermembrane space)
CoQ carries two electrons and
yields one electron to two
different cytochrome c molecules
via the Q cycle, which also is the
mechanism for complex III to
pump protons into the
intermembrane space.
The antifungal agent stigmatellin
inhibits electron flow from CoQH2
CoQH2 + 2 cytochrome c1 (Fe3+) + 2 H+ (matrix)
CoQ + 2 cytochrome c1 (Fe2+) + 4 H+ (intermembrane space)
The final cytochrome in the ETC is cytochrome c which
moves electrons from Complex III to Complex IV
Conserved Lys
residues (blue balls)
play a key role in
coordinating the
cytochrome with
different proteins
Complex IV reduces oxygen to water
Complex IV =
cytochrome c
oxidase
4 cytochrome c (Fe2+) + 8 H+ (matrix) + O2
4 cytochrome c (Fe3+) + 2 H2O + 4 H+ (intermembrane space)
A CuA center and CuB atom
interacting with a couple of
cytochrome heme iron atoms
The proximity of all
those redox species
allows this sequence
of redox reactions to
occur
There are two protontranslocating
channels that allow
movement into the
intermembrane space
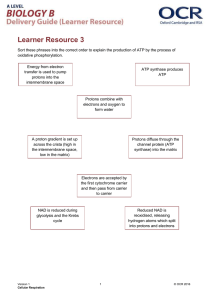
The coupling of the ETC and ATP synthesis
(oxidative phosphorylation)
By 1960, it was known that oxidative phosphorylation
was the manner in which NADH transferred its energy
to generate ATP; the question was how?
For OP to occur, the inner mitochondrial membrane needed to be
intact, and that the membrane was impermeable to most ions,
including H+
Chemiosmotic theory
“The free energy of electron transport is conserved by
pumping H+ from the mitochondrial matrix to the
intermembrane space to create an electrochemical H+
gradient across the inner mitochondrial membrane. The
electrochemical potential of this gradient is harnessed to
synthesize ATP.”
Mitchell, P., “Coupling of phosphorylation to electron and
hydrogen transfer by a chemiosmotic type of mechanism”,
Nature (1961), 191: 144-148.
Protonmotive force (pmf)
The free energy generated by the H+ electrochemical gradient
ΔG = 2.3 RT {pH (side 1) – pH (side 2)} + Z F ΔΨ
Okay, this is going to take some explaining…
Protonmotive force (pmf)
The free energy generated by the electrochemical gradient
ΔG = 2.3 RT {pH (matrix) – pH (outside)} + Z F ΔΨ
Okay, this is going to take some explaining…
There are two parts to the
generation of free energy:
1. “pH (matrix) – pH (outside)” or
ΔpH is the energy simply due to
the concentration gradient of H+
2. “ΔΨ” is the energy due to the
potential difference brought
about by the separation of charge
Protonmotive force (pmf)
The free energy generated by the electrochemical gradient
ΔG = 2.3 RT {pH (matrix) – pH (outside)} + Z F ΔΨ
Okay, this is going to take some explaining…
Z = the normalized charge on a
proton = +1
F = Faraday’s constant = 96485
J/V
ΔΨ = membrane potential, which
is positive when a proton is
moved from a negative region to a
positive region
Evolutionarily, bacteria (which don’t have mitochonria) and
mitochondria are related in the mechanism of oxidative
phosphorylation/electron transport. Bacteria use enzymes
similar to Complexes I through IV on their plasma membrane to
perform the OP/ET.
Making ATP: ATP synthase = proton-pumping
ATP synthase = F1F0-ATPase
Multisubunit transmembrane
protein, 450 kD
F1 subunit projecting into the matrix
F1 component of ATP
synthase has a subunit
composition of α3β3γδε
γ is a “stalk” that is 114 Å
in length, and acts like an
axle to the α3β3
“segments”. There is
pseudo-threefold
symmetry because each αβ
unit has a slightly different
conformation: E = empty
(distorted binding site); DP
= binds ADP; TP = binds
ATP (only the β subunit
participates in ATP
synthesis).
Though the αβ protomers are centered on the γ
subunit, the “cap” is not connected to the “axle”
In fact, the ε subunit
anchors the γ subunit to
the F0 subunit.
F0 subunit
has an ab2c1015 structure.
The c subunit
F1
ring is F0’s
defining
feature; it is
the proton
translocator.
F0
Each of the αβ protomers in F1 have three possible conformations:
O = open (inactive), L = loosely-binding (inactive), T = tightlybinding (active), so ATP is generated only in the T conformation
protomer.
In step 2 of the mechanism above, there is a conformational shift
caused as energy is released from the proton gradient and “turns”
the γε axle (green). The c ring (not shown) accepts protons and
moves them along the gradient to the matrix side of the
membrane; this force turns the c ring that turns the γε subunits.
To put it all together:
If there are 10 c subunits then 10 H+
= 1 full turn of the c ring
= 3 ATP made
The c, γ and ε subunits
rotate; the rest of the
subunits do not move,
though they may change
conformation. a and c
subunits translocate
protons; the αβ protomers
synthesize ATP. The b2
and δ subunits act to
prevent the αβ protomers
from rotating; a subunit
may act as a ratchet to
prevent the c ring from
rotating the wrong way.
To demonstrate that the c ring turns under a proton
gradient, this clever experiment was performed
The αβ protomers were
adhered to a glass slide via
His residues. On the other
end, a fluorescent-labeled
actin fiber was attached to
the c ring via a streptavidin
molecule. Then an ATP
solution was added onto
the glass slide.
What resulted was the apparent counter-clockwise motion of the
actin filament consistently over most of the adhered ATPases.
Sambongi Y., Iko, Y., Tanabe, M., Omote, H., Iwamoto-Kihara,
A., Ueda, I., Yanagida, T., Wada, Y. and Futai, M. (1999)
Mechanical rotation of the c subunit oligomer in ATP synthase
(F0F1): direct observation. Science, 286: 1722–1724
The P/O ratio measures the number of ATP molecules
synthesized per oxygen molecule reduced.
This molecule donates an electron pair to Complex IV and reduces
an oxygen molecule to water, translocating 2 H+ in the process and
turning the c ring one-third of a turn. Thus its P/O ratio is 1.
The P/O ratio for NADH (and other electron carriers that enter the
ETC at Complex I) is 2.5 (10 protons translocated); for FADH2
(and other carriers that enter at Complex II), it is 1.5 (6 protons
translocated).
Uncouplers decouple the ETC from oxidative phosphorylation
(and thus ATP synthesis) by facilitating the free diffusion of
protons across the inner membrane.
This means that all of the free energy
of the electron carriers is given off as
heat: non-shivering thermogenesis
(Case Study 23).
Regulation of oxidative phosphorylation is
controlled by [NADH]/[NAD+] and ATP
UCP1 is a proton channel protein that equilibrates the
mitochondrial proton gradient; it is only found in brown adipose
tissue.
A complex
regulatory
cascade
governs this
protein
channel!
Aerobic metabolism has advantages over anaerobic (32
ATP vs 2 ATP) but some disadvantages (oxidative damage
to tissues)
The enzyme
superoxide dismutase
converts any
superoxide radical
generated by
oxidative
phosphorylation to
hydrogen peroxide