Initial Investment in CDISC Data Standards
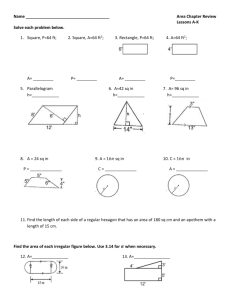
in Clinical Organisations
Knitting fog, herding cats, and other
challenging endeavours
Ken Stoltzfus
Octagon Research Solutions
PhUSE 2010 Berlin
1
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
About Octagon
• Octagon Research Solutions
– A development partnering organization that offers regulatory,
clinical, process and IT solutions to the life sciences industry.
• Octagon experience on topic:
–
–
–
–
600 studies converted to SDTM & ADaM (8,000+ domains)
Current CDISC SDS team lead
2 of 6 co-authors of CDISC SDTM
Current CDISC team membership: SDS, ADaM, Trial
Design, Controlled Terminology, and XML
– CDISC-authorized SDTM trainers
2
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Agenda
•
•
•
•
•
•
3
Background
Standards Governance
People
Standards
Processes
Technical Solutions
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Background
4
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards
Who needs them ????
5
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards
Success story !!!!
6
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Value
• Example: Analysis and Submission
– Integrated standards provide traceability when the
regulatory reviewer has questions
– Data mining for safety signals facilitated by
adherence to a common standard structure
– Emerging regulatory standards yield reviews with
decreased review time and increased data
accuracy
7
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Metrics
Impact of CDISC Standards Implementation
on Clinical Study Cycle Time & Cost1
Clinical
Study
Activities
Current
Industry
Average
(months)
After CDISC
Standards
Time
Implementation Savings
(months)
(months)
Cost
Savings
(USD
Million)
Study Start-up
5
1
4
4.4
Study Conduct
4
2.4
1.6
1.8
Analysis &
Reporting
5
2.5
2.5
2.8
Cumulative
14
5.9
8.1
9
1
8
“Business Case for CDISC Standards: Full Report, PhRMA-Gartner-CDISC Project” (May 2007), http://www.cdisc.org
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Obstacles
• Acknowledging the fears
–
–
–
–
–
9
Inhibiting scientific innovation
Perception of standards governance as bureaucracy
Business needs compete with standards needs
Unacceptable cost in time and resources
The standards will not be stable
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards Governance
10
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards Governance
• What exactly is standards governance?
–
–
–
–
–
–
11
Oversight
Decision-making
Development
Maintenance
Compliance
Enforcement
Solution:
Standards
Governance
Framework
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards Governance
Standards Governance Framework
• Standards – Governance = No standards at all
– Irregular, non-compliant usage
– Eventually non-existent
• Minimum framework:
– Roles & responsibilities
– Development resources
– Business guidelines
• Maximum framework:
– Processes
– Tools
12
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards Governance
• People
• Standards
• Processes
Leadership
Developers
Content decision-makers Data stewards
Internal standards
CDISC standards
Interpretation
Development
Business Guidelines
Maintenance
SOPs
• Technical Solutions
Request system
CDR
13
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
MDR
People
14
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
People
• Numbers
– Varies from company to company
– Part-time resources far outnumber the full-time ones
• Full-time
– Team leader(s)
– Decision-makers (content & structure)
• Part-time
–
–
–
–
15
Oncology physician for RECIST tumor measurements
EDC screen developer including monitoring needs in eCRF
Statistical programmer writing standard macro code
IT rep as liaison - governance group to IT project managers
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
People
• Content decision-makers
– Subject matter experts (medical, clinical, statistical)
• Developers
– Technical expertise
– Operational managers
• Administrators
– Tracking requests
– Updating metadata
– Uploading the latest version
• Data Stewards
– TAs
– Functional areas
16
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
People
• High-level directive that creates buy-in
–
–
–
–
–
–
–
Improved data quality
Improved data transparency
Accurate, consistent submissions
Speed of study execution
Maximization of study execution resources
Cost savings
Compliance with anticipated regulatory
requirements
– Keeping up with competitors who are becoming
more streamlined
17
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
People
• Definition of standards drivers
– Bridge gap between the strategic & operational
– Working knowledge of standards (doesn’t have to
be a standards expert)
– A champion for standards in their functional area
18
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards
19
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards
• What are Standards ????
–
–
–
–
A company’s methodology in a stable format
Intellectual property in some cases
Internal vs external
Reusable pieces used in clinical trials
•
•
•
•
•
•
•
20
Data definition files
eCRF screens
EDC data extracts
Data checking utilities/programs
Macros for standard results
Analysis dataset programs
XML transfer files
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards
• 80 / 20 rule
80: Of the total content that a
function delivers for a study, 80%
should come from quick, efficient,
20: Only 20% of total content that a
reusable standards
function delivers for a study should
come from customized or newly
developed standards
Custom
Reusable
21
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards
• Levels to facilitate management of standards
– Global
– Therapeutic Areas
– Project
Custom
Reusable
22
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Standards
• SDTM implementation considerations
–
–
–
–
–
–
–
–
–
23
SDTM Findings class in collection: horizontal vs vertical
SDTM mapping: stability of standard CRFs
Best location in the data lifecycle for conversion to SDTM
SDTM experience in the EDC functional area
Linear implementation: CDASH to SDTM to ADaM
Which databases house data needed to build Trial Design?
SDTM & ADaM metadata
Define.xml: How? Who?
SDTM training: Who? What? When?
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Processes
24
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Processes
• Governance charter
–
–
–
–
–
25
Strategic
Structure
Organisation
Roles & Responsibilities
Scope
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Processes
• Standard Operating Procedures (SOPs)
–
–
–
–
–
–
26
Development of standards
Maintenance of standards
Request process for new or revised standards
Role of the data steward in governance
Access control for standards storage
Waiver process
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Processes
• Business Guidelines for CDISC Standards
– Mapping decisions
– Common practices
– Interpretation
27
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Technical Solutions
28
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Technical Solutions
• Do they really help ???
• Technical solutions that make a difference
– Standards request system
– Clinical data repository (CDR)
– Metadata repository (MDR)
29
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Technical Solutions
• Standards Request System
–
–
–
–
–
–
The face of the standards governance framework
Allow study teams to submit standards requests
Allow for supportive files
Assign unique identification number
Track requests by date, resolution, user, etc.
Fulfill request with discussions, questions, and
decisions
– Provide users with status of their requests
– Provide reporting capabilities (metrics, etc.)
30
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Technical Solutions
• Clinical Data Repository
–
–
–
–
Repose all data in standard centralized location
Import, storage, and export of data in SDTM
Validated inbound ETL process
Promote standardized, validated data import and
export
– Support the production of analysis datasets and
clinical study reports
– Interoperability with clinical systems across the
clinical data architecture
31
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Technical Solutions
• Metadata Repository
– Repose all metadata in standard centralized
location
– Repose study definition metadata
– Ensure areas for all standards (global, TA, project)
– Import and export standards metadata
– Interoperability with clinical systems across the
clinical data architecture
– Generate governance process workflows
– Maintain proper version control, data traceability &
audit trails
32
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Summary
33
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
• Standards Governance
–
–
–
–
34
People
Standards
Processes
Technical Solutions
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
• Spend time thinking up-front
35
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
• Avoid herding cats
36
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Acknowledgements
Barry Cohen
Dave Evans
Tore Haglund
Stephen Harrison
Harpreet Sahni
37
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Contact Information
Ken Stoltzfus
Clinical Data Strategies
Octagon Research Solutions
585 East Swedesford Road, Suite 200
Wayne, PA 19087
USA
Tel: 1-610-535-6500 (Ext. 5891)
kstoltzfus@octagonresearch.com
38
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.
Questions
?
39
© 2010 Octagon Research Solutions, Inc. All Rights Reserved.