RESEARCH WHICH CAN BE EXEMPTED FROM FULL IRB REVIEW
advertisement

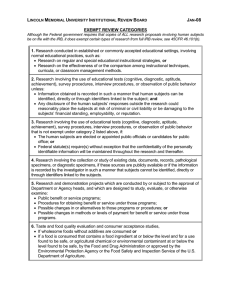
NUS Institutional Review Board (IRB) RESEARCH WHICH CAN BE EXEMPTED FROM FULL IRB REVIEW All Principal Investigators who wish to apply for exemption from full IRB review for their research proposals must write in officially to the Chairman, NUS-IRB using the IRB Exemption Form (IRB-Form-008). The NUS-IRB will evaluate the application for exemption and inform the researcher of its decision. The Board reserves its discretion to make the final decision. In the absence of local legislation, the following categories have been adopted from the US OHRP Guidelines 45 CFR 46.101 as best practice. Unless otherwise required by the Faculty or Department heads, research activities in which the only involvement of human subjects will be in one or more of the following categories are exempted from full IRB review: (1) Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as (i) research on regular and special education instructional strategies, or (ii) research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods. *(2) Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behaviour, unless: (i) information obtained is recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subjects; and (ii) any disclosure of the human subjects' responses outside the research could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects' financial standing, employability, or reputation. (3) Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, or observation of public behaviour that is not exempted under paragraph (2) of this section, if: (i) the human subjects are elected or appointed public officials or candidates for public office; or (ii) Federal statute(s) require(s) without exception that the confidentiality of the personally identifiable information will be maintained throughout the research and thereafter. (4) Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. (5) Research and demonstration projects which are conducted by or subject to the approval of Department or Agency heads, and which are designed to study, evaluate, or otherwise examine: (i) Public benefit or service programs; (ii) procedures for obtaining benefits or services under those programs; (iii) possible changes in or alternatives to those programs or procedures; or (iv) possible changes in methods or levels of payment for benefits or services under those programs. (6) Taste and food quality evaluation and consumer acceptance studies, (i) if wholesome foods without additives are consumed or (ii) if a food is consumed that contains a food ingredient at or below the level and for a use found to be safe, or agricultural chemical or environmental contaminant at or below the level found to be safe, by the US Food and Drug Administration or approved by the US Environmental Protection Agency, or equivalent agencies in Singapore. * Please refer to overleaf for the Explanatory Notes. References: OHRP Guidelines 45 CFR 46.101 IRB-GUIDE-006 Page 1 of 2 Version No: 4 Date of Revision: 27 Jan 2014 Explanatory Notes to IRB-GUIDE-006 1. There will be no exemption when deception of the subjects is an element in the research or if the research involves vulnerable populations such as children, prisoners, persons with cognitive impairments etc. 2. The NUS-IRB recognizes that some research may involve more than minimal risk even in the absence of subject identifiers. For example, anonymous survey may contain invasive questions that may cause the subject to experience emotional distress or discomfort when answering them. Thus, although the research technically qualifies for exempt status because there are no subject identifiers, the potential risks of the research should negate exemption 1. 3. According to Singapore BAC’s Guidelines for IRBs on Research Involving Human Subjects2, the following categories of Indirect Human Biomedical Research could also be considered for Exemption from full IRB review, taking current practice into account: (a) Writing up or reporting of individual patients’ clinical results by the patients’ doctors, provided that the patients’ consent for procedures and interventions in clinical management have been obtained and the patients’ privacy protected. Researchers who are not the attending physicians in the programme but wish to have access to such information should send their proposals to the IRB in the usual way; (b) Research using appropriately designed data escrow or other arrangements in which personal or other identity information is securely withheld from researchers by a third party provider of information, there being no possibility of researchers by themselves being able to trace or reconstruct significant information on the identity of subject donor; (c) The development of diagnostic tests using existing samples for test validation purposes provided that the necessary consent for the taking and use of the samples has been obtained. References: 1 Bankert, E. & Amdur, R. Institutional Review Board: Management and Function, Second Edition. Sudbury, MA: Jones and Bartlett Learning, 2006 2 Singapore BAC’s Guidelines for IRBs on Research Involving Human Subjects, November 2004 IRB-GUIDE-006 Page 2 of 2 Version No: 4 Date of Revision: 27 Jan 2014