CHAPER 4 Thermodynamics of Fossil, Biomass, and Synthetic Fuels
advertisement

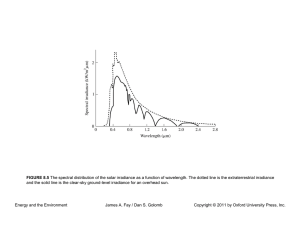
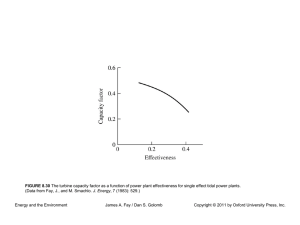
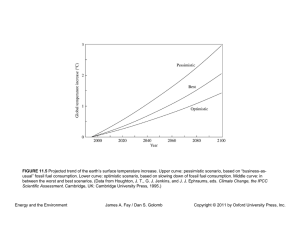
CHAPER 4 Thermodynamics of Fossil, Biomass, and Synthetic Fuels Energy and the Environment James A. Fay / Dan S. Golomb Copyright © 2011 by Oxford University Press, Inc. FIGURE 4.1 A sketch of a hydrogen–oxygen fuel cell (not to scale). Hydrogen and oxygen are supplied to porous electrodes separated by an electrolyte in which the electric current is carried by hydrogen ions. In the external electric circuit, current is carried by a matching electron flow. The product of oxidation, water, evolves from the cathode. Energy and the Environment James A. Fay / Dan S. Golomb Copyright © 2011 by Oxford University Press, Inc. FIGURE 4.2 Idealized sketches of (a) fuel cell voltage and power as a function of current and (b) efficiency as a function of power. Energy and the Environment James A. Fay / Dan S. Golomb Copyright © 2011 by Oxford University Press, Inc. FIGURE 4.3 A plot of typical fuel cell performance showing the dimensionless electrode potential Δ/(Δ)th and electrical power output IΔ /Iss(Δ)th versus the dimensionless current I/Iss. Energy and the Environment James A. Fay / Dan S. Golomb Copyright © 2011 by Oxford University Press, Inc.