Methane
advertisement

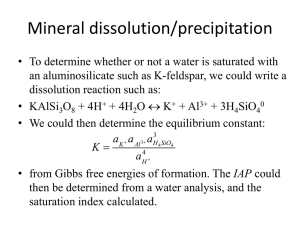
Methane • CH4 • Greenhouse gas (~20x more powerful than CO2) • Formed biologically (methanogenesis) • Huge reservoir as methane clathrate hydrate in cold soils and ocean bottom – stable structure at low T, high P • 2x1016 kg of C in these deposits • What happens if the oceans warm?? • ‘Clathrate gun’ hyothesis – warming seas ‘melt’ these clathrates, CH4 released en masse to atmosphere… Microbes and methane production • Methanogenesis – Reduction of CO2 or other organics to form CH4 (also CH4 generation from special fermentative rxns) – Only certain groups of Archaea do this, specifically with the Euryachaeota subdivision – Called methanogens • These organisms do not compete well with other anaerobes for e- donors, thus they thrive where other alternate e- acceptors have been consumed Methane cycle Microbial methane oxidation • Organisms that can oxidize CH4 are Methanotrophs – mostly bacteria • All aerobic methanotrophs use the enzyme methane monooxygenase (MMO) to turn CH4 into methanol (CH3OH) which is subsequently oxidized into formaldehyde (HCHO) on the way to CO2 • Anaerobic methane oxidation – use SO42- as the e- acceptor – this was long recognized chemically, but only very recently have these microbes been more positively identified (though not cultured) Phosphorus cycle • P exists in several redox states (-3, 0, +3, +5) but only +5, PO43-, stable in water • 1 microbe to date has been shown to grow on PO33- (phosphite, P3+) as a substrate • P is a critical nutrient for growth, often a limiting nutrient in rivers and lakes • Most P present as the mineral apatite (Ca5(PO4)3(F,Cl,OH)); also vivianite (Fe3(PO4)2*8H2O) P sorption • P strongly sorbs to FeOOH and AlOOH mineral surfaces as well as some clays • P mobility thus inherently linked to Fe cycling • P sorption to AlOOH is taken advantage of as a treatment of eutrophic lakes with excess P (alum is a form AlOOH) – AlOOH is not affected by microbial reduction as FeOOH can be. P cycling linked to SRB-IRB-MRB activity PO43- PO43- PO4 Org C + SO42- FeS2 FeOOH H2S PO43- 3- PO43- Sulfate Reducers PO43- PO43- PO43Blue Green Algae blooms Redox ‘Fronts’ Oxic Anoxic • Boundary between oxygen-rich (oxic) and more reduced (anoxic) waters • Oxygen consumed by microbes which eat organic material • When Oxygen is gone, there are species of microbes that can ‘breathe’ oxidized forms of iron, manganese, and sulfur St. Albans Bay Sediments Mn2+ + 2e- --> Mn0(Hg) H2O2 + 2e- + 2H+ 2H2O O2 + 2e- + 2H+ H2O2 Fe3+ + 1e- Fe2+ FeS(aq) 7-19-04 Core 1 Profile 1 10 10 5 5 0 0 -5 -5 Mn (nA) -10 O2 (nA) -15 Fe3+ (nA) -20 -20 FeS (nA) -25 -25 -30 -30 -10 O2 (nA) -15 Depth (mm) Depth (mm) 6-23-04 Core 2 Profile 2 -35 -35 0 20 40 60 80 0 Current (nA) 10 5 5 0 0 -5 -5 -15 Mn (nA) Depth (mm) Depth (mm) 10 O2 (nA) 100 8-12-04 Core 1 Profile 1 7-26-04 Core 2 Profile 1 -10 50 Current (nA) -10 -15 O2 (nA) -20 -20 Mn (nA) -25 -25 FeS (nA) -30 -30 Fe3+ (nA) -35 -35 0 20 40 60 Current (nA) 80 0 20 40 60 Current (nA) 80 Results: Seasonal Work • Sediments generally become more reduced as summer progresses • Redox fronts move up and down in response to Temperature, wind, biological activity changes Seasonal Phosphorus mobility Depth 0-1 cm 1-2 cm 2-3 cm 3-4 cm 4-5 cm 5-6 cm 6-8 cm 8-10 cm Ascorbic Acid P-Fe P-Mn 0.863 0.894 0.933 0.921 0.829 0.567 0.604 0.559 0.732 0.777 0.889 0.895 0.866 0.804 0.894 0.876 Profiles seasonal sample averages 0 Depth (top of section, cm) 1 2 AA P 3 AA Fe / 10 4 AA Mn 5 NaOH P 6 7 8 9 0 100 200 Conc. (mg/g sediment) 300 • Ascorbic acid extractions of Fe, Mn, and P from 10 sediment cores collected in summer 2004 show strong dependence between P and Mn or Fe • Further, profiles show overall enrichment of all 3 parameters in upper sections of sediment • Fe and Mn would be primarily in the form of Fe and Mn oxyhydroxide minerals transformation of these minerals is key to P movement P Loading and sediment deposition Profiles seasonal sample averages 0 • Constantly moving redox fronts affect Fe and Mn minerals, mobilize P and turn ideal profile into what we actually see… Depth (top of section, cm) 1 2 AA P 3 AA Fe / 10 4 AA Mn 5 NaOH P 6 7 8 9 0 100 200 Conc. (mg/g sediment) 300