CRYSTALLINITY AS A PART OF PREFORMULATION STUDY
advertisement

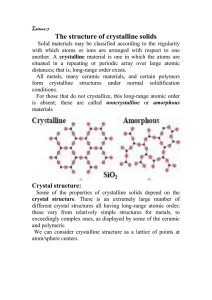
CRYSTALLINITY AS A PART OF PREFORMULATION STUDY CONTENTS INTRODUCTION CLASSIFICATION OF SOLIDS AMORPHOUS POLYMORPHS SOLVATES CLATHRATES COMPARISON OF CRYSTALLINE AND AMORPHOUS FORMS CRYSTAL STRUCTURE AND MORPHOLOGY MODIFICATION OF CRYSTAL HABIT AND ITS CHARACTERIZATION CRYSTALLIZATION ANALYTICAL METHOD FOR CHARACTERIZATION IMPORTANCE IN PREFORMULATION STUDIES LATEST TECHNIQUE DEVELOPMENTS REFERENCES STUDY QUESTIONS 2 INTRODUCTION A crystal is a solid in which the constituent atoms, molecules, or ions are packed in a regularly ordered, repeating pattern extending in all three spatial dimensions. The study of the crystalline form as a part of preformulation studies is termed as crystallinity studies. 3 CLASSIFICATION OF CHEMICAL COMPOUND Solids Crystalline Amorphous (Non-crystalline) Single entity Polymorphs Enantiotropic Monotropic Molecular adducts Non-stoichiometric Inclusion compounds Channel Layer Cage (Clathrate) Stoichiometric compounds Hydrates Solvates 4 AMORPHOUS COMPOUND They have atoms or molecules randomly placed as in a liquid. They are typically prepared by: Lyophilization. E.g. Fluprednisolone in tert-butanol. Rapid quenching of chloramphenicol palmitate solution in hydrophilic solvent. Rapid quenching of melted chloramphenicol palmitate in the refrigerator to -10◦ Precipitation is also used to prepare the amorphous prompt insulin zinc suspension. 5 Amorphous forms are of : Higher thermodynamic energy Greater solubility and dissolution rate. But due to high energy they are unstable and revert back to a stable form. Eg. Amorphous novibiocin suspension. Agents like methylcellulose, polyvinylpyrollidone, and several alginic acid derivatives such as sodium alginate and propylene glycol algin are used to prevent such condition. 6 POWDER X-RAY DIFFRACTION PATTERN 7 POLYMORPHS Many drug substances can exist in more than one crystalline form with different space-lattice arrangements. This phenomenon is known as polymorphism and the different crystalline forms as polymorphs. Drugs like barbiturates have polymorphic forms. Also steroid hormones have 42 and sulphonamides have 30 polymorphic forms. 8 SOLVATES (PSEUDOPOLYMORPHISM) Solvates are molecular complexes that have incorporated the crystallizing solvent molecule in their specific lattice position and in fixed stoichiometry. Estradiol forms highest number of solvates with all 30 solvents. Other eg are erythromycin, chloramphenicol, ampicillin, sulphanilamide etc. Solvates can be distinguished from polymorphs by observing bubbles of gas in silicon oil upon heating. 9 CLATHRATES A clathrate is a single-phased solid with two distinct components: the host and the guest. The guest is retained in the closed cavities provided by the crystalline structure of the host. Thus it is a non-stoichiometric molecular adduct. The major classes of clathrates are hydroquinone clathrates, water clathrates, phenol clathrates etc. 10 PHARMACEUTICAL APPLICATIONS OF CLATHRATES:PURIFICATIONSEPARATION OF RARE GASES- SEPARATION OF OPTICAL ISOMERSSTORAGE OF INERT GASES- MODE OF ACTION OF ANESTHETICS[ JPS-1975, 64, 1264.] 11 COMPARISON OF THE MECHANICAL PROPERTIES OF THE COMPACTS OF THE CRYSTALLINE AND AMORPHOUS FORMS OF A DRUG SUBSTANCE CRYSTALLINE FORM AMORPHOUS FORM More ductile (low indentation hardness value) Least ductile (high indentation hardness value) Form compacts with highest tensile strength Compacts have low brittleness value Form compacts with lowest tensile strength Require higher compression stress Require lower compression stress Compacts have high brittleness value 12 COMPARISON OF SOLUBILITY OF CRYSTAL, SOLVATE AND HYDRATE: Amorphous form more soluble than a corresponding crystalline form. The dissolution rates of hydrates are less than corresponding anhydrous crystalline form. E.g gluthethimide, theophylline, caffeine, succinyl sulphathiazole, phenobarbitol. The dissolution rates for organic solvates are higher than corresponding pure crystaline form. E.g. 1,4-dioxane solvate of nifedipine shows better solubility than dihydrate form. So organic solvates should be preferred in place of pure crystals which solves both problems, solubility and stability, but only if ICH guidelines about limits of organic residues permit. 13 Crystals are of two types:Irregularly shaped crystals known as anhedral or allotriomorphic. Definite shaped crystals bound by plane faces known as euhedral or idiomorphic. Any crystal is characterized by its internal structure and habit. Habit is the description of the outer appearance of a crystal whereas the internal structure is the molecular arrangement within the solid. 14 CRYSTAL SYSTEM (INTERNAL STRUCTURE) The most symmetric system is cubic system. Other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic. Thus there are fourteen types of unit cell called as the Bravais lattices. We can identify the various planes of crystal using the system of Miller indices. 15 CRYSTAL HABIT There are five types of crystal habit widely recognized: Platy: plates Tabular: moderate expansion of two parallel faces Prismatic: columns Acicular: needle-like Bladed: flat acicular These occur in all the seven systems. 16 Crystal habit can be quantitatively expressed in terms of aspect ratio (AR). AR defined as the ratio of length to width and values of AR approaching 1 (spherical or cube shape) are considered to be pharmaceutically good. It is preferable to keep the AR values below 5 so as to avoid problems with flow. AR in polar solvents was as high as 9.4 in comparison with 5-6 in non-polar solvents. [JPP-2007, 59, 29-39.] 17 METHODS OF MODIFICATIONS OF CRYSTAL HABIT Excessive supersaturation. E.g. transform a prism or isodiametric crystals to needle shape. Cooling rate and agitation. E.g. naphthalene gives thin plates if rapidly cooled whereas slow evaporation yields prisms. The crystallizing solvent. E.g. resorcinol produces needles from benzene and squat prisms from butyl acetate. Addition of co-solvents or solutes. E.g. sodium chloride is cubic but urea produces octahedral habit. Crystal habit can also be modified by adding impurities or ‘poisons’; for example, sulphonic acid dyes alter the crystal habit of ammonium, sodium and potassium nitrates. 18 CHARACTERIZATION OF HABITS The angle between two crystals faces can be described in two ways: Included or edge angle between two faces, Interfacial or polar angle, the angle between the normals to the faces of the crystal. Interfacial angle is of importance in crystallography. They are measured by instruments known as goniometers. 19 CRYSTALLIZATION Crystallization is a chemical solid-liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. The crystallization process consists of two major events: Nucleation Homogenous Heterogenous Crystal growth 20 Supersaturation is the driving force of the crystallization This can be achieved by various methods, with 1) solution cooling, 2) addition of a second solvent to reduce the solubility of the solute (technique known as anti-solvent or drown-out) 3) chemical reaction 4) change in pH being the most common methods used in industrial practice. Other methods, such as solvent evaporation, can also be used. 21 ANALYTICAL METHODS FOR CHARACTERIZATION OF SOLID FORMS METHOD MATERIAL REQUIRED per SAMPLE 1mg 1mg Microscopy Fusion methods (hot stage microscopy) Differntial scanning calorimetry (DSC/DTA) Infrared spectoscopy X-ray powder diffraction Scanning electron microscopy Thermogravimetric analysis Dissolution/solubility analysis 2-5mg 2-20mg 500mg 2mg 10mg mg to gm 22 IMPORTANCE OF CRYSTALLINITY IN PREFORMULATION STUDIES EFFECT ON SOLUBILITY & BIOAVAILABILITY Antibiotic novobiocin is inactive in crystyalline form while amorphous form has 10 times more solubility and hence more bioavailable. 23 EFFECT ON INSULIN NO TYPE OF INSULIN . FORM OF INSULIN ONSET OF ACTION DURATION OF ACTION 1 Prompt insulinzinc suspension (semilente) Amorphous fast Short 2 Extended insulin-zinc suspension (ultralente) Insulin-zinc suspension (lente) crystalline slow Long 3 30%amorphou fast s+ 70%crystalline Intermediate 24 CHEMICAL STABILITY At instances crystalline form are more stable than amorphous form. e.g. crystalline forms of penicillin G as potassium or sodium salt are more stable. SUSPENSION SYRINGEABILITY A suspension of plate shaped crystals may be injected through a needle with a greater ease than one with needle shaped crystals of same dimensions. 25 EFFECT ON GRANULATION Sulphathiazole can exist in different crystalline forms . Form III has water adsorption of 0.046 mg/m2 & form I has water adsorption of 0.031 mg/m2 so form III shows better wetting and so easy granulation. Use of amorphous form of calcium pentothenate in multivitamin tablets prepared by wet granulation process, is not desirable because polymorphic transformation makes the granulation mass sticky, making futher granulation virtually impossible. 26 HARDNESS OF TABLET Sulphamerazine is available in two different crystalline forms SMZ-I & SMZ-II. SMZ-I forms harder tablets than SMZ-II at same compression pressure and so it shows delayed release. Both these forms can be used in single tablet by compression coating in which the core is formed of SMZ-I and coat is made up of SMZ-II to get repeat action. 27 EFFECT ON CONSOLIDATION Substances possessing the cubic lattice arrangement were tabletted more satisfactorily than those with rhombohedral lattice. The isotropic nature of former group contribute to better tabletting because no alignment of particular lattice planes is required. In addition provide three equal planes for stress relief at right angles to each other. 28 DIRECTLY COMPRESSIBLE EXCIPIENTS The DC grade excipients are microgranulations, since they consist of masses of small crystallites randomly embedded in a matrix of glue-like (often amorphous) material. Such a combination imparts the desired overall qualities which results in strong tablet by providing a plastically deforming component (the matrix) to relieve internal stresses and strongly bonding surfaces (the faces of crystallites) to enhance consolidation. 29 POLYMORPHIC TRANSFORMATION Many drugs undergo polymorphic transformation during various processes. E.g. during grinding drugs like digoxin, estradiol, spironolactone, phenylbutazone undergo transformation. By granulation of theophylline with water converts into monohydrate from anhydrous form. Similarly by drying and compression also drugs undergo change in their form. 30 LATEST TECHNIQUE DEVELOPMENTS IN CRYSTALLIZATION SPHERICAL CRYSTALLIZATION It has been developed by Yoshiaki and co-workers. It is a solvent exchange crystallization method in which crystal agglomeration is purposefully induced through the addition of third solvent termed as “Bridging liquid” which act as granulating agent. 31 It is a novel technique to improve compressibility, good flowability and bioavailability of pharmaceuticals. Moreover tablets formed have greater mechanical strength and lower friability. Various drugs have been successfully undergone this process to acquire improved micromeritic properties and thus have shown increased dissolution rate like salicylic acid, mefenemic acid, aminophylline, tolbutamide. [Pharmaceutical Research-1994, 11(4)]32 METHODS OF SPHERICAL CRYSTALLIZATION SIMPLE SPHERICAL CRYSTALLIZATION E.g. spherical crystallization of salicylic acid from ethanol by addition of water, using chloroform as bridging unit. QUASI-EMULSION-SOLVENT-DIFFUSION METHOD E.g. antirheumatic drug bucillamine was crystallized as spheres by this method using HPMC. Also controlled release microspheres of ibuprofen with acrylic polymers was accomplished by this method. AMMONIA DIFFUSION METHOD Useful for amphoteric drugs like enoxacin. NEUTRALIZATION METHOD Tolbutamide dissolved in sodium hydroxide and HPEC aqueous solution was crystallized using this method. 33 CONTROLLED CRYSTALLIZATION Very useful method for getting microcrystals in very narrow size range for hydrophobic drugs. E.g. anti-inflammatory drug betamethasone dipropionate, triamcinolone acetonide, beclomethasone. [JPS-2003, 92] AMORPHOUS FORM STABILIZATION NEUSILIN (amorphous magnesium aluminium silicate) was milled in the ball mill with the drugs like indomethacin, ketoprofen,naproxen and progesterone. The amorphous form thus formed was more stable than normal amorphous form and did not turned to crystalline form easily. Thus by using additives with high glass transition temperature or by selective hydrogen bonding with the stabilizing additives conversion of amorphous form to crystalline form can be prevented. 34 [JPS-2003, 92(3)] SUPER CRITICAL FLUID CRYSTALLIZATION It is a novel technique used for selective production of polymorphs and pseudopolymorphs from aqueous solution. Glycine has three polymorphs and can be selectively precipitated to either pure α- or β-glycine by this technique. [Chemical Abstract, (2007), 147(12), 1058:258004m] CHIRAL DRUGS Resolution of chiral drugs and drug intermediate is done by preferential crystallization. 35 [Chemical Abstract, (2007), 146(25), 1729:507024v] CONCLUSION Thus, with all examples of the effects of habits, polymorphs, solvates and clathrates on optimising pharmaceutical formulations, the crystal chemistry has become a routine part of every pharmaceutical company’s preformulation programme. 36 REFERENCES Pharm. Dosage forms and drug delivery system , ANSEL, 100,151. Pharm. Dosage forms, LACHMANN and LIBERMANN, 1, 26-30. Modern pharmaceutics, BANKER,MARSHALL DEKKER INC. Pharm Encyclopedia, 3, 399. Pharm Encyclopedia, 12, 320-321. Advanced pharmaceutical solids, CARSTENSEN, 110, 6. Physical pharmacy, ALFRED MARTIN. Industrial pharmacy, LACHMANN and LIBERMANN. Physico-chemical principles of pharmacy, A.T.FLORENCE & D.ATTWOOD, 8-10. 37 REFERENCES Pharmaceutics-the science of dosage form design, M.E.AULTON, 142- Pharmaceutical sciences, REMINGTON, 1358. Journ. Of Pharm. Sciences, (2007), 96, 990. Journ. Of Pharm. Sciences, (2006), 95, 26-30. Journ. Of Pharm. Sciences, (2006), 95, 446. Journ. Of Pharm. Sciences, (2006), 95, 1641. Journ. Of Pharm. Sciences, (2003), 92, 35-46. Journ. Of Pharm. Sciences, (1989), 78, 68-72. Journ. Of Pharm. Sciences, (1987), 76, 471-474. 149. 38 REFERENCES Journ. Of Pharm. Sciences, (1984), 73, 1407-1410. Journ. Of Pharm. Sciences, (1975), 64, 1264. Journ. Of Pharm. Sciences, (1963), 52, 781-791. Journ. Of Pharmaceutics and Pharmacology, (1975), 28, 94. Pharmaceutical research, (1994), 11. Int. Journ.Of Pharm., (2002), 241, 73-85. Advanced Drug Delivery Reviews, (2007), 59, 617-630. Chemical Abstract, (2007), 147(12), 1058. Chemical Abstract, (2007), 146(25), 1729. 39 STUDY QUESTIONS 1) Explain factors affecting crystal habit & its pharma applications. (1st internal 2005) 2)Crystallization is inhibited by PVP – Discuss. (1st internal 2005) 3) Compare crystal and amorphous. How solubilities of crystal hydrate & solvate differ? (1st internal 2006) 4) Define: spherical crystallization. (1st internal 2006) 5) Write a note on spherical crystallization. (August 2006) 6) Write a note on crystallinity? 7) What are clathrates? Give its pharmaceutical applications? 40 41