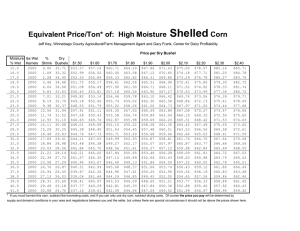
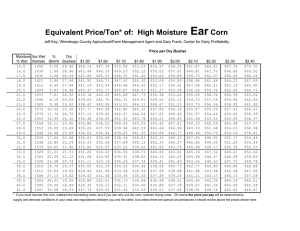
– Unit 8 Activity General Chemistry Counting Particles
advertisement

Name Date Pd General Chemistry – Unit 8 Activity Counting Particles 1. Observe the beakers of corn and rice on the lab station. Make a guess of how many kernels of corn and grains of rice are in the beakers. Record your guess. Guessed number of corn kernels __________ Guessed number of rice grains __________ 2. Determine the mass of 25 corn kernels and 25 rice grains. Mass of 25 kernels __________ Mass of 25 grains __________ 3. Based on the information in question #2, propose a method for estimating the number of corn kernels and rice grains in their respective beakers. Write a brief procedure for this, including plans for calculations, in the space below. 4. Carry out the procedure from question #3, showing all work below. If you need any additional equipment, ask your instructor. Estimated number of kernels of corn __________ Estimated number of grains of rice __________ Modeling Chemistry 1 U8 gen Counting Particles 5. Count the actual number of kernels of corn in your beaker and record below. Actual number of kernels of corn __________ 6. Compare the estimated value with the actual amount of corn and rice that was in each beaker by using the percent error equation. Show your work below then comment on the ability to accurately estimate the amount of large quantities of very small objects. 7. Based on the data collected in this activity, how would the mass of 525 kernels of corn compare to the mass of 525 grains of rice? Mathematically validate your response. 8. How would the number of particles compare in 10.0 g of rice as compared to 10.0 g of corn? Mathematically validate your response. 9. In chemistry we deal with very small particles, far smaller than even a grain of rice or kernel of corn. Write a conclusion for this activity relating the techniques used to determine the number of grains in each beaker to how chemists might determine the number of atoms in 42.5g of pure gold. Modeling Chemistry 2 U8 gen Counting Particles