OLS Specific Airway Resistance in Young Children
advertisement

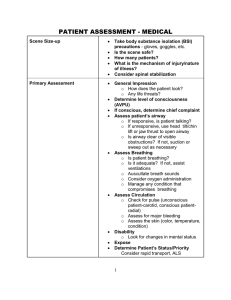
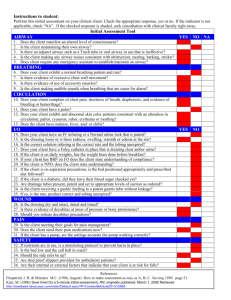
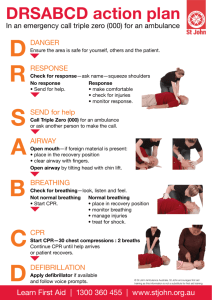
OLS Specific Airway Resistance in Young Children Reference Equations for Specific Airway Resistance in Children: The Asthma UK Initiative: The Online Data Supplement: Authors: J Kirkby, S Stanojevic, L Welsh, S Lum, M Badier, C Beardsmore, A Custovic, K Nielsen, J Paton, W Tomalak, J Stocks Calculation of specific airways Resistance sRaw: Derivation of sRaw can be calculated from the relationship of plethysmographic box pressure (Pbox) to flow during spontaneous breathing, and in the simplest form can be derived from the tangent of the slope of Pressure/Flow. Derivation of equation[1]: sRaw is the product of Raw and FRC: 1) Raw = (Vbox_spontaneous / flow) / (Vbox_occlusion / Pmouth) 2) FRC = (Vbox_occlusion / Pmouth) x (Pamb – PH20) These 2 equations can be combined: 3) sRaw = Vbox_spontaneous / flow ∙ Vbox_occlusion ∙ (Pamb-PH2O) Vbox_occlusion / Pmouth Pmouth And simplified, thereby avoiding need for an airway occlusion with which to calibrate box pressure changes in terms of alveolar pressure changes. 4) sRaw = Vbox_spontaneous / flow ∙ (Pamb-PH2O) Where Vbox_spontaneous = change in box volume during spontaneous breathing Vbox_occlusion = change in box volume during efforts against the airway occlusion (shutter) Pmouth = change in mouth (alveolar) pressure during efforts against the airway occlusion Pamb = ambient pressure PH2O = water vapour pressure Kirkby et al, January 2010 1 OLS Specific Airway Resistance in Young Children However since variations of airway resistance occur during a single quiet breath, a sRaw outcome which is calculated as a regression of pressure and flow over the entire breathing cycle i.e. ‘effective resistance’ (sReff) may be more informative of changes in breathing mechanics. The Integration method is unique to Jaeger plethysmographs and calculates sReff from multiple points throughout the breathing cycle and values are averaged over four breaths. sReff is the ratio of work of breathing to the square of the effective flow[2]. Thus the areas of box pressure vs. flow and mouth pressure vs. flow are integrated and divided with consideration of calibration factors (e.g. BTPS correction). The equation in its simplest form is: sReff = Reff ∙ (FRCbox + VT/2) [2, 3] Thus computation of pressure and flow can be taken throughout the entire breathing cycle and resistance can be calculated as follows: sReff = (Pamb * Integral ∆VdV)) / Integral V’dV where: Pamb is the ambient pressure; Integral ∆VdV is equivalent to the area enclosed by the specific work of breathing loop, and integral V’dV is equivalent to the area of the flow / volume-loop. Description of statistical techniques: LMS is an extension of regression analysis which includes three components: 1) the skewness (Lambda), which models the departure of the variables from normality using a Box-Cox transformation, 2) the median (Mu), and 3) the coefficient of variation (Sigma), which models the spread of values around the median and adjusts for any non-uniform dispersion, hence LMS. The coefficient of variation (CV) is defined as (100 x Kirkby et al, January 2010 2 OLS Specific Airway Resistance in Young Children SD/median). The three quantities (LMS) are allowed to change with height and/or age, to reflect changes in the distribution as children grow. We applied the LMS method using the Generalized Additive Models of Location Scale and Shape (GAMLSS) package in the statistical program R (Version 2.6.1; R Foundation, http://www.r-project.org)[4] Separate models were developed for males and females. Fractional polynomials were used to produce smoothly changing curves, which explain the age related changes. The goodness of fit was assessed using the Schwarz Bayesian Criterion (SBC) which compares consecutive models directly while adjusting for the increased complexity to determine the simplest model with best fit. A detailed description of the GAMLSS technique as it pertains to spirometry can be found in Cole et al.(2009)[5] Methodological differences: Where differences in methodology were observed, subanalyses were conducted to establish the impact of these differences: 1) Filters: Two centres routinely used bacterial filters, one only used filters for specific patient groups e.g. cystic fibrosis. To establish the impact of using bacterial filters, paired sets of sRaw measurements were obtained in adults using a mouthpiece and noseclip with and without a bacterial filter in situ at a breathing frequency of 30bpm within a 10 minute interval. The equipment was calibrated with a filter in situ and the “filter check box” within the software was checked for all measurements i.e. the software had an internal adjustment that corrected for the added resistance created by the filter at all times, therefore the absolute difference of not having a filter in situ could be measured. All measurements were undertaken by the same operator, and analysed by a Kirkby et al, January 2010 3 OLS Specific Airway Resistance in Young Children different operator, masked to measurement conditions. Results: sRtot was significantly higher when filters were used; mean (95%CI) difference: 0.19kPa∙s (0.13; 0.25) at 30bpm (p=0.0001). With an average FRC of 3.1L in these subjects, this equates to a change in Raw of 0.06kPa∙L -1.s, which is in keeping with the manufacturer’s claim that bacterial filters add no more than 0.1 kPa∙L -1.s to the resistance (Air Safety LTD, Lancs, UK). 2) Breathing Frequency: Measurements with and without the filter in situ were repeated on the same occasion at 80bpm to elucidate the influence of breathing rate and flow on sRaw. Results: An increase in breathing frequency from 30bpm to 80bpm was accompanied by an increase in both flows (mean (95% CI) increase: 0.75L/s (0.36; 1.13)), and sRaw (mean (95%CI) difference: 0.26kPa∙s (0.43; 0.95)) 3) Quality Control Over-read: See Appendix for sRaw over-read sheet. Table E1 summarises the details of the over-read score from the three centres that provided sufficient data for this exercise. One of these centres did not include breathing frequency on the P/F curve print-outs, but confirmed that the protocol always maintained breathing frequency between 30-45bpm, so were scored appropriately for this criteria. Table E1: Summary of QC scores for sRaw loops from the three included centres. Site Resp. Rate Super-imposable Similar size Closed at No >1 set Over-all (n) (30-45 bpm) breaths and shape zero flow distortion availableb score. 2 (10) 8 (80%) 9 (90%) 5 (50%) 6 (60%) 7 (70%) 10 (100%) 5/6 4 (70) 70 (100%) 65 (93%) 60 (86%) 59 (84%) 59 (84%) 70 (100%) 5/6 5 (18) 100 (100%)a 18 (100%) 7 (39%) 0 (0%) 11 (6%) 18 (100%) 3/6 Kirkby et al, January 2010 4 OLS Specific Airway Resistance in Young Children Footnote: Results represent the number (%) of subjects from each centre that achieved each QC criteria, and the average over-all score for the two centres that supplied original P/F curves for over-reading. a Reported to maintain all recordings within 30-45bpm, but not recorded on printouts. b i.e. at least 2 technically acceptable trials. Kirkby et al, January 2010 5 OLS Specific Airway Resistance in Young Children Box 1: Recommendations for sRaw data collection in children Demographics: The following test details should be recorded whenever collecting reference data: Measured standing height (+ sitting height for healthy controls): Recorded to nearest 0.5cm Calculated age: Recorded as (date of test) – (date of birth), in years to 1 decimal place. Measured weight : Recorded to the nearest 0.5kg Sex: recorded and coded consistently Gestational age: Recorded as completed weeks Birth weight: Recorded to nearest 0.1kg Ethnicity: Consensus required for definition Equipment: Make, model and software version needs to be recorded. o A biological validation should be performed prior to upgrading software or equipment Use an appropriately sized mouthpiece and noseclip. (a modified mask may also be used if that is current practice, though many centres now report equal success with a mouthpiece) Always use a bacterial filter o Ensure calibrations are performed with filter in situ, and internal settings have accounted for the filter. Data Collection Ensure the child is sitting upright, with no leak between the lips and mouthpiece Cheek supported with child’s hands. o NB, while not strictly necessary if no occlusion manoeuvres are performed, this is good practice for when plethysmographic assessments include measures of FRC. Natural breathing pattern within the specified range of 30-45bpm, avoiding either shallow panting or hyperventilation. Collect at least 3 sets of ‘technically acceptable’ data during with no gross distortion. Quality Control: Use the automatic computer selected tangent. Use the sRaw over-read sheet in the appendix to “grade” the quality of the measurement. sRaw Outcomes: Report sReff as the main outcome measure o sRtot should be recorded where available, to facilitate comparisons with published data and provide objective evidence regarding most discriminative outcome in future clinical trials o Breathing frequency, peak expiratory and inspiratory flow (PEF and PIF respectively) should be recorded as QC outcomes, Report the Median of the median: Select the median (middle) breath from the median trial of 3 technically acceptable sets of 5 or 10 breaths Footnote : An automated method of measuring sRaw over the central linear portion of the breath in a manner that is independent of the child’s age[6] would be a useful addition, but has yet to be implemented by manufacturers Kirkby et al, January 2010 6 OLS Specific Airway Resistance in Young Children APPENDIX: SPECIFIC AIRWAY RESISTANCE (sRaw) OVER-READ INFORMATION: Data collection: Performed from age > 3yrs For accurate measurements of sRaw to be obtained it is essential that: Prior to commencing test, sufficient time (at least 60 seconds) is allowed for thermal stabilisation with the subject sitting in the plethysmograph. Subject must wear a nose-clip / nose must be occluded Cheeks are supported with hands. Subject is encouraged to perform regular, quiet tidal breathing (30-45 bpm). o Rapid deep breathing which can lead to hyperinflation and high peak flows should be discouraged Manual amendments to the pressure-flow loops should not be made. Up to 5 trials of 10 or 5 breaths (dependent on software) should be recorded with the aim of obtaining 3 ‘technically acceptable’ trials, as defined below. Quality Control Score: A trial of sRaw loops can graded according to the following criteria (Y = 1, N = 0): 1. Respiratory rate is between 30-45 bpm Y/N 2. Breaths are super-imposable (i.e. parallel slopes) Y/N 3. Breaths are of similar size and shape Y/N 4. Breaths are reasonably closed at zero flow Y/N 5. There are no obvious distortions to the breath Y/N (e.g. glottic closure, cough, talking) 6. There is more than one acceptable trial available Y/N See www.growinglungs.org.uk for examples, and evidence on which these recommendations are based. Kirkby et al, January 2010 7 OLS Specific Airway Resistance in Young Children REFERENCES: 1. Dab I, Alexander F. A simplified approach to the measurement of specific airway resistance. Pediatr Res 1976: 10(12): 998-999. 2. Jaeger MJ, Otis AB. Measurement of Airway Resistance with a Volume Displacement Body Plethysmograph. J Appl Physiol 1964: 19: 813-820. 3. Manzke H, Stadlober E, Schellauf HP. Combined body plethysmographic, spirometric and flow volume reference values for male and female children aged 6 to 16 years obtained from "hospital normals". Eur J Pediatr 2001: 160(5): 300-306. 4. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992: 11(10): 1305-1319. 5. Cole TJ, Stanojevic S, Stocks J, Coates AL, Hankinson JL, Wade AM. Age- and size-related reference ranges: a case study of spirometry through childhood and adulthood. Stat Med 2009: 28(5): 880-898. 6. Stocks J, Thomson A, Silverman M. The numerical analysis of pressure-flow curves in infancy. Pediatr Pulmonol 1985: 1(1): 19-26. Kirkby et al, January 2010 8