Document 15532367
advertisement

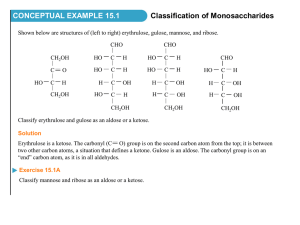
03-131 Genes, Drugs, and Disease Lecture 24 October 27, 2015 Concept Map: 1. Carbohydrates 2. Lipids & Membranes 3. Metabolism - pathways Glycolysis Electron transport ATP synthesis Anaerobic metabolism (fermentation) 4. Cholesterol metabolism & cholesterol control 5. Cell Signaling and breast cancer. Introduction to Carbohydrates: Monosaccharides: All carbons in monosaccharides are 'hydrated' -hence the name carbohydrate (general formula (CH2O)N). Each carbon is bound to one oxygen. The first or the second carbon is a C=O. 1. The simplest monosaccharides contain three carbons (dihydroxyacetone, glyceraldehydes) 2. When the C=O group is at the 2nd position it's called an ketose, because the functional group is a ketone, e.g. dihydroxyacetone. 3. When the C=O group is at the very beginning it's an aldose, because the functional group is an aldehyde, e.g. glyceraldehydes. 4. Additional hydrated carbons (HO-C-H) are added just below the aldehyde or ketone group to make longer carbohydrates. 5. The added carbon generates a new chiral center. Each aldose differs from its neighbor by the configuration (orientation of –OH) of at least one of the carbon. dihydroxyacetone (no chiral center) D-glyceraldehyde Aldoses D-Glyceraldehyde Ribose Glucose Ketoses: The addition of three (HO-C-H) units to dihydroxyacetone gives the 6 carbon ketose - fructose, an important sugar in metabolism. Fructose Aldose – C=O on carbon one. Ketose – C=O on carbon two All other carbons have –OH group. 1 03-131 Genes, Drugs, and Disease Lecture 24 October 27, 2015 Ring Formation in Glucose 1. Six membered ring created by forming a bond between C1 and O5. Most stable state. 2. The C1 carbon becomes chiral and is called the anomeric carbon 3. The new OH group (on C1) can exist in either the or form. α-down β-up Ring formation in Ribose Formation of a 5 membered ring can occur by forming a bond between C1 and O4. H CH2OH O CH2OH O H O H O OH H OH H OH H O OH OH OH CH2OH H O H OH C6 Ketose: Fructose - Although this is a 6-carbon sugar, because it is a ketose a five membered ring is formed when it forms a ring. CH2OH O O CH2OH OH OH H OH OH OH H H O OH 1 CH2OH C C OH C 2 4 2 C CH2OH CH2OH 5 O 3 C O CH2OH O HO C OH HO HO 3 H 4 H 5 C C OH H OH O C O CH2OH H CH2OH CH2OH 5 C 4 CH2OH C OH C 2 O OH O HO CH2OH 3 C OH OH Disaccharides: Linkage of the anomeric carbon of one monosaccharide to the OH of another monosaccharide via a condensation reaction. The bond is termed a glycosidic bond. At least on anomeric carbon participates in forming the bond. glucose glucose maltose Lactose (milk sugar): Major sugar in mammalian milk. Infants produce lactase to hydrolyze the disaccharide to monosaccharides, the released glucose and galactose are sued Some adults have low levels of lactase. This leads to lactose intolerance. The ingested lactose is fermented by bacteria in the large intestine, producing uncomfortable volumes of CO2. CH2OH O CH2OH O HO CH2OH OH OH O OH OH glucose OH galactose CH2OH O HO OH O O OH OH OH OH (alternate drawing) 2 03-131 Genes, Drugs, and Disease Lecture 24 October 27, 2015 Sucrose (table sugar): The anomeric carbon of glucose forms a glycosidic bond to the anomeric carbon of fructose. Sucrose C. Polysaccharides: Many monosaccharides linked by glycosidic bonds. Most poly-saccharides are polymers of either glucose, or modified glucose. Short-hand nomenclature: In the case of homo-polymers, the short-hand notation is to simply describe the linkage between the glucose units: both the conformation of the anomeric carbon and the carbons participating in the glycosidic bond, i.e. (1-4). Energy Storage Polysaccharides: Glucose is released when required. glucose fructose 1. Starch [plants] (mixture of amylose and amylopectin). amylose = (1-4) glucose. . 2. Amylopectin [plants] = amylose plus (1-6) branches. CH2OH CH2OH O O CH2OH O O O O O O CH2OH OH 3. Glycogen [animals] = more highly branched than amylopectin. glycogen starch Structural Polysaccharides 1. Cellulose: Structural polysaccharide of plants. 1-4 glucose, can't be digested by mammalian enzymes. Digested by symbiotic microorganisms (such as those in termites) CH2OH O CH2OH O (alternative drawings) CH2OH O O OH OH (1-4) O OH OH O O CH2OH O O O O OH CH2OH CH2OH CH2OH OH O OH OH 2. Bacterial Cell Walls (Peptidoglycan) Polysaccharide chains of alternating N-acetylglucose amine (NAG) and Nacetylmuraminc acid (NAM) Muramic acid on NAM linked to a small peptide (L-Ala D-Gln L-Lys DAla). NAM peptide chains are crosslinked with penta(5)glycine bridges that extend off of terminal Ala and join to L-Lys sidechain on adjacent chain, forming a tough crosslinked cell wall. O OH O OH O (1-4) OH O OH OH CH2OH O OH OH OH CH2OH O OH H3C CH2OH CH2OH O OH O O OH O OH OH OH OH OH HN NH2 glucosamine CH3 O N-acetyl glucosamine (NAG) OH HN CH3 O N-acetylmuramic acid (NAM) 3 03-131 Genes, Drugs, and Disease Lecture 24 October 27, 2015 NAM NAG NAM NAG Mechanism of Action of Penicillin: Penicillin inhibits enzymes that are responsible for crosslinking the Gly5 chain to alanine (circled on diagram). The crosslinking of the cell wall is broken, making the bacteria fragile to breakage. Inhibition is by formation of a chemical bond between penicillin and the enzyme (covalent inhibitor). This type of inhibition is not penicillin the same as competitive inhibition because: i) the penicillin forms a covalent bond with the enzyme, ii) penicillin is modified by the reaction, iii) the reaction is essentially irreversible. H H H S CH 3 R O N H CH3 COOH S CH 3 R N O O H Serine H H H CH3 COOH H H S CH 3 R N O O H CH3 COOH N O O H S CH 3 R O R CH3 COOH H Serine S CH 3 N O CH3 COOH OH H O Serine Serine H H OH H Serine Penicillin Resistance: Bacterial produce a protein that degrades penicillin (β-lactamase). This is a common antibiotic resistance gene that is used on plasmids. The transformed bacterial are resistant to penicillin! 4