EXAMPLE#1---- CH 13 # 50 in “Zummie” PROBLEM OBJECTIVES
advertisement
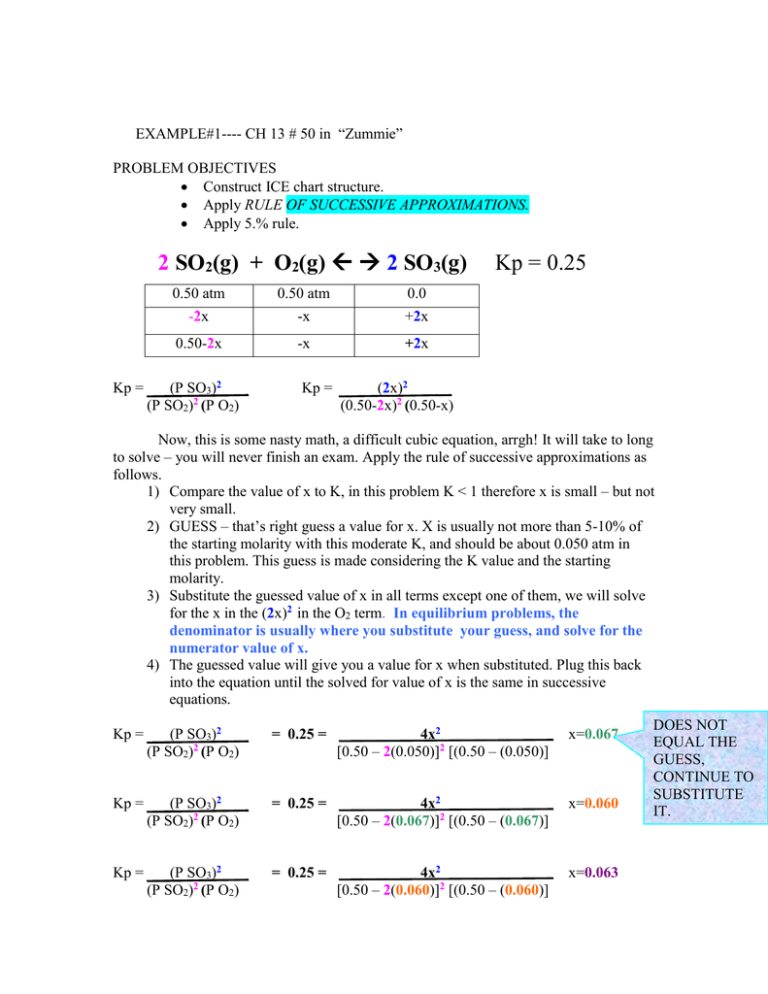
EXAMPLE#1---- CH 13 # 50 in “Zummie” PROBLEM OBJECTIVES Construct ICE chart structure. Apply RULE OF SUCCESSIVE APPROXIMATIONS. Apply 5.% rule. 2 SO2(g) + O2(g) 2 SO3(g) Kp = 0.50 atm -2x 0.50 atm -x 0.0 +2x 0.50-2x -x +2x (P SO3)2 (P SO2)2 (P O2) Kp = Kp = 0.25 (2x)2 (0.50-2x)2 (0.50-x) Now, this is some nasty math, a difficult cubic equation, arrgh! It will take to long to solve – you will never finish an exam. Apply the rule of successive approximations as follows. 1) Compare the value of x to K, in this problem K < 1 therefore x is small – but not very small. 2) GUESS – that’s right guess a value for x. X is usually not more than 5-10% of the starting molarity with this moderate K, and should be about 0.050 atm in this problem. This guess is made considering the K value and the starting molarity. 3) Substitute the guessed value of x in all terms except one of them, we will solve for the x in the (2x)2 in the O2 term. In equilibrium problems, the denominator is usually where you substitute your guess, and solve for the numerator value of x. 4) The guessed value will give you a value for x when substituted. Plug this back into the equation until the solved for value of x is the same in successive equations. Kp = (P SO3)2 (P SO2)2 (P O2) = 0.25 = 4x2 [0.50 – 2(0.050)]2 [(0.50 – (0.050)] x=0.067 Kp = (P SO3)2 (P SO2)2 (P O2) = 0.25 = 4x2 [0.50 – 2(0.067)]2 [(0.50 – (0.067)] x=0.060 Kp = (P SO3)2 (P SO2)2 (P O2) = 0.25 = 4x2 [0.50 – 2(0.060)]2 [(0.50 – (0.060)] x=0.063 DOES NOT EQUAL THE GUESS, CONTINUE TO SUBSTITUTE IT. Kp = (P SO3)2 (P SO2)2 (P O2) = 0.25 = 4x2 [0.50 – 2(0.063)]2 [(0.50 – (0.063)] x=0.062 THIS VALUE RETURNS ITSELF WHEN IT IS SUBSTITUTED, SO IT IS THE VALUE WE GO WITH.