11.2
Types of Chemical Reactions
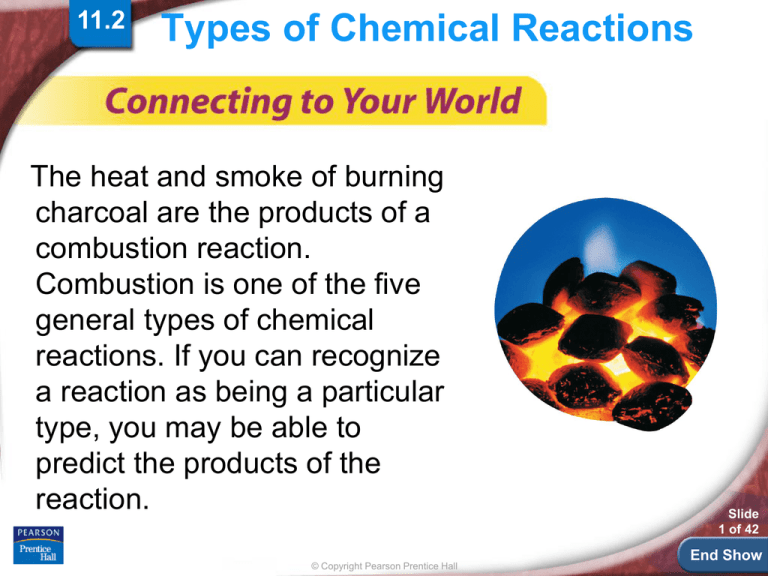
The heat and smoke of burning
charcoal are the products of a
combustion reaction.
Combustion is one of the five
general types of chemical
reactions. If you can recognize
a reaction as being a particular
type, you may be able to
predict the products of the
reaction.
© Copyright Pearson Prentice Hall
Slide
1 of 42
End Show
11.2
Classifying Reactions
Types of Chemical Reactions
>
Five general types of chemical reactions:
•
combination (synthesis)
•
decomposition
•
single-replacement
•
double-replacement
•
combustion
See websites for videos and animations
http://www.youtube.com/watch?v=i-HHvx1VC_8&NR=1
http://www.youtube.com/watch?v=tE4668aarck&feature=related
Slide
2 of 42
© Copyright Pearson Prentice Hall
End Show
11.2
Classifying Reactions
Combination Reactions
A combination reaction is a chemical change
in which two or more substances react to form a
single new substance.
for Conceptual Problem 11.4
11.2
Types of Chemical Reactions
>
Classifying Reactions
Decomposition Reactions
A decomposition reaction is a chemical
change in which a single compound breaks
down into two or more simpler products.
Slide
6 of 42
© Copyright Pearson Prentice Hall
End Show
Slide
7 of 42
© Copyright Pearson Prentice Hall
End Show
Slide
8 of 42
© Copyright Pearson Prentice Hall
End Show
Slide
9 of 42
© Copyright Pearson Prentice Hall
End Show
for Conceptual Problem 11.5
11.2
Types of Chemical Reactions
>
Classifying Reactions
Single-Replacement Reactions
A single-replacement reaction is a chemical
change in which one element replaces a second
element in a compound.
Slide
11 of 42
© Copyright Pearson Prentice Hall
End Show
11.2
Types of Chemical Reactions
>
Classifying Reactions
The activity series of metals
lists metals in order of
decreasing reactivity.
For a single-replacement reaction to
occur, the element that is displaced
must be less active than the element
that is doing the displacing.
Metals above Hydrogen in the activity
series will replace H from acids.
Zn(s) + 2 HCl (aq) → H2 (g) + ZnCl2 (aq)
Metals below Hydrogen in the activity
series will not react with acids.
Slide
12 of 42
© Copyright Pearson Prentice Hall
End Show
Slide
13 of 42
© Copyright Pearson Prentice Hall
End Show
Slide
14 of 42
© Copyright Pearson Prentice Hall
End Show
Slide
15 of 42
© Copyright Pearson Prentice Hall
End Show
for Conceptual Problem 11.6
11.2
Types of Chemical Reactions
>
Classifying Reactions
Double-Replacement Reactions
A double-replacement reaction is a chemical
change involving an exchange of positive ions
between two compounds.
Slide
17 of 42
© Copyright Pearson Prentice Hall
End Show
11.2
Types of Chemical Reactions
>
Classifying Reactions
Combustion Reactions (see regents ref tables)
A combustion reaction is a chemical change in
which an element or a compound reacts with
oxygen, often producing energy in the form of
heat and light.
Slide
21 of 42
© Copyright Pearson Prentice Hall
End Show
Slide
22 of 42
© Copyright Pearson Prentice Hall
End Show
Slide
23 of 42
© Copyright Pearson Prentice Hall
End Show
Note
your regents reference tables are helpful
for writing equations for combustion
reactions
Examples:
Slide
24 of 42
© Copyright Pearson Prentice Hall
End Show
11.2
Types of Chemical Reactions
>
Predicting the Products of a
Chemical Reaction
Predicting the Products of a Chemical
Reaction
How can you predict the products of the
five general types of reactions?
The number of elements and/or compounds
reacting is a good indicator of possible
reaction type and thus possible products.
Slide
25 of 42
© Copyright Pearson Prentice Hall
End Show
11.2
Types of Chemical Reactions
>
Predicting the Products of a
Chemical Reaction
Slide
26 of 42
© Copyright Pearson Prentice Hall
End Show
11.2
Types of Chemical Reactions
>
Predicting the Products of a
Chemical Reaction
Slide
27 of 42
© Copyright Pearson Prentice Hall
End Show
11.2
Types of Chemical Reactions
>
Predicting the Products of a
Chemical Reaction
Slide
28 of 42
© Copyright Pearson Prentice Hall
End Show
11.2
Types of Chemical Reactions
>
Predicting the Products of a
Chemical Reaction
Slide
29 of 42
© Copyright Pearson Prentice Hall
End Show
11.2
Types of Chemical Reactions
>
Predicting the Products of a
Chemical Reaction
Slide
30 of 42
© Copyright Pearson Prentice Hall
End Show
11.2 Section Quiz.
Assess students’ understanding
of the concepts in Section 11.2.
Continue to:
-or-
Launch:
Section Quiz
Slide
31 of 42
© Copyright Pearson Prentice Hall
End Show
11.2 Section Quiz.
1. What type of reaction is described by the
following equation?
6Li + N2 2Li3N
a. combination reaction
b. decomposition reaction
c. single-replacement reaction
d. combustion reaction
Slide
32 of 42
© Copyright Pearson Prentice Hall
End Show
11.2 Section Quiz.
2.
Balance the following equation and indicate whether it
represents a combustion, combination, or decomposition
reaction.
H2SO4 H2O2 + SO2
a. H2SO4 H2O2 + SO2, combination reaction
b. H2SO4 H2O2 + SO2, decomposition reaction
c. H2SO4 2H2O2 + SO2, combination reaction
d. H2SO4 2H2O2 + SO2, decomposition reaction
Slide
33 of 42
© Copyright Pearson Prentice Hall
End Show
11.3
Reactions in Aqueous Solution
Structures in limestone caverns
are formed when carbon
dioxide converts calcium
hydrogen carbonate into
calcium carbonate. The calcium
carbonate precipitates and
forms dramatic stalactites and
stalagmites. You will learn to
predict the formation of
precipitates and write
equations to describe the
reactions that produce them.
Slide
34 of 42
© Copyright Pearson Prentice Hall
End Show
11.3
When AgNO3 (aq) and NaCl (aq) are mixed, a white
precipitate of AgCl (s) is formed. We can write a balanced
equation to describe this double-replacement reaction:
AgNO3 (aq) + NaCl (aq) → AgCl (s) + NaNO3 (aq)
Two other types of equations can be written for this reaction
(complete ionic equation and a net-ionic equation)
A complete ionic equation is an equation that shows
dissolved ionic compounds as dissociated free ions.
Slide
35 of 42
© Copyright Pearson Prentice Hall
End Show
11.3
Net Ionic Equations
An ion that appears on both sides of an
equation and is not directly involved in the
reaction is called a spectator ion.
The net ionic equation is an equation for a
reaction in solution that shows only those
particles that are directly involved in the
chemical change.
Slide
36 of 42
© Copyright Pearson Prentice Hall
End Show
11.3
Net Ionic Equations
Complete ionic equation:
Sodium ions and nitrate ions are not changed
during the chemical reaction of silver
nitrate and sodium chloride so the net
ionic equation is
Slide
37 of 42
© Copyright Pearson Prentice Hall
End Show
11.3
Predicting the Formation of a Precipitate
Predicting the Formation of a Precipitate
How can you predict the formation of a precipitate
in a double-replacement reaction?
You can predict the formation of a precipitate by
using the general rules for solubility of ionic
compounds and by using the regents reference
tables.
Slide
38 of 42
© Copyright Pearson Prentice Hall
End Show
11.3
Predicting the Formation of a Precipitate
Will a precipitate form when a sodium
carbonate solution is mixed with a barium
nitrate solution?
Hints: what are the products? and are those
products soluble? (Use your reference
tables).
Slide
39 of 42
© Copyright Pearson Prentice Hall
End Show
11.3
Predicting the Formation of a Precipitate
Balanced equation:
Ba(NO3)2 + Na2CO3 (aq)→ BaCO3 (s) + 2 NaNO3 (aq)
Complete ionic equation:
Ba2+(aq) + 2 NO3 - (aq) + 2 Na+(aq) + CO3 2- (aq)
→
BaCO3(s) + 2 Na+(aq)+ 2 NO3 - (aq)
Net Ionic equation:
Slide
40 of 42
© Copyright Pearson Prentice Hall
End Show
11.3 Section Quiz.
2. Which one of the following products of
double-replacement reactions would NOT
form a precipitate?
a. AgCl
b. PbSO4
c. Mg(OH)2
d. Mo(NO3)2
Slide
41 of 42
© Copyright Pearson Prentice Hall
End Show
11.3 Section Quiz
3. Which reaction will NOT produce a precipitate
from aqueous solution?
a. Hg2(NO3)2 + KCl
b. FeSO4 + Ba(OH)2
c. Pb(NO3)2 + Na2CO3
d. NaBr + Al2(SO4)2
Slide
42 of 42
© Copyright Pearson Prentice Hall
End Show