2.2. pp
advertisement

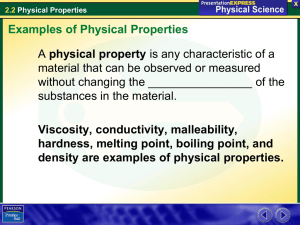
2.2 Physical Properties There are pitchers of ice water and lemonade on a picnic table. How do you know which liquid is in each pitcher? It’s easy! Lemonade is yellow and has a tart taste that is hard to miss. A yellow color and a tart taste are two properties of lemonade. 2.2 Physical Properties Examples of Physical Properties What are some examples of physical properties? A physical property is any characteristic of a material that can be observed or measured without changing the composition of the substances in the material. Viscosity, conductivity, malleability, hardness, melting point, boiling point, and density are examples of physical properties. 2.2 Physical Properties Examples of Physical Properties Viscosity The tendency of a liquid to keep from flowing is called its viscosity. • Thick liquids, such as corn syrup and honey, have a high viscosity. • Thin liquids, such as vinegar and water, have a low viscosity. 2.2 Physical Properties Examples of Physical Properties Conductivity A material’s ability to allow heat to flow is called conductivity. • Materials that have a high conductivity, such as metals, are called conductors. • Good conductors of heat are usually also good conductors of electricity. 2.2 Physical Properties Examples of Physical Properties Malleability The ability of a solid to be hammered without shattering is malleability. • Most metals, such as gold, are malleable. • An ice cube or piece of glass breaks into small pieces when struck with a hammer. Solids that shatter when struck are brittle, not malleable. 2.2 Physical Properties Examples of Physical Properties Hardness One material can scratch another material if it is harder than the other material. • A kitchen knife can scratch a copper sheet because stainless steel is harder than copper. • The material used to sharpen the knife blade must be harder than stainless steel. Diamond is the hardest known material. 2.2 Physical Properties Examples of Physical Properties This Tlingit carver is using an adze to carve a canoe from Western red cedar. Red cedar is a relatively soft wood. The adze is hard. 2.2 Physical Properties Examples of Physical Properties Melting and Boiling Points The temperature at which a material changes state is a physical property. • The temperature at which a substance changes from solid to liquid (melts) is its melting point. • The temperature at which a substance changes from liquid to gas (boils) is its boiling point. 2.2 Physical Properties Examples of Physical Properties Melting and Boiling Points Which of these substances are liquids at room temperature (20C, or 68F)? Answer: 2.2 Physical Properties Examples of Physical Properties Melting and Boiling Points Which of these substances are liquids at room temperature (20C, or 68F)? Answer: octane, water, and acetic acid 2.2 Physical Properties Examples of Physical Properties Density The ratio of the mass of a substance to its volume is its density. • Density can be used to test the purity of a substance. • Silver has a density of 10.5 g/cm3. A coin with a density of 9.9 g/cm3 is not made from silver, or it contains substances in addition to silver. 2.2 Physical Properties Using Physical Properties How can knowing the physical properties of matter be useful? Physical properties are used to identify a material, to choose a material for a specific purpose, or to separate the substances in a mixture. 2.2 Physical Properties Using Physical Properties Using Properties to Identify Materials A material can be identified by its properties. • Decide which properties to test. • Do tests on a sample of the unknown material. • Compare the results with the data reported for known materials. 2.2 Physical Properties Using Physical Properties Using Properties to Choose Materials Properties determine which materials are chosen for which uses. • For example, shoelaces must be flexible, that is they must be able to bend without breaking. • They must also be durable, that is, they must be able to withstand repeated use. 2.2 Physical Properties Using Physical Properties Laces in hiking boots are usually made of nylon or leather, not from wood. 2.2 Physical Properties Using Properties to Separate Mixtures What processes are used to separate mixtures? Filtration and distillation are two common separation methods. 2.2 Physical Properties Using Properties to Separate Mixtures Filtration You can separate hot tea from loose tea leaves by pouring the mixture through a strainer. Filtration is a process that separates materials based on the size of their particles. 2.2 Physical Properties Using Properties to Separate Mixtures These students filter (sift) dirt through a wire screen to locate small objects. Particles of dirt are small enough to pass through the holes, but objects such as broken bits of pottery are too large. 2.2 Physical Properties Using Properties to Separate Mixtures Distillation Sometimes all the particles in a solution are small enough to pass through a filter. Distillation is a process that separates the substances in a solution based on their boiling points. 2.2 Physical Properties Recognizing Physical Changes The change of water from a liquid to a gas during boiling is a physical change. A physical change occurs when some of the properties of a material change, but the substances in the material remain the same. 2.2 Physical Properties Recognizing Physical Changes During a physical change, the size and shape of a material can change but not the composition. Some examples include • melting butter in a pan • crumpling a piece of paper • slicing a tomato 2.2 Physical Properties Recognizing Physical Changes Some but not all physical changes can be reversed. Braiding hair is a reversible change. Cutting hair cannot be reversed. 2.2 Physical Properties Assessment Questions 1. Which of the following is not a physical property? a. b. c. d. density boiling point flammability conductivity 2.2 Physical Properties Assessment Questions 1. Which of the following is not a physical property? a. b. c. d. density boiling point flammability conductivity ANS: C 2.2 Physical Properties Assessment Questions 2. Which of these materials is not malleable? a. b. c. d. copper aluminum glass gold 2.2 Physical Properties Assessment Questions 2. Which of these materials is not malleable? a. b. c. d. copper aluminum glass gold ANS: C 2.2 Physical Properties Assessment Questions 3. In choosing a material for use as a wire to carry electric current, which physical property would be most important? a. b. c. d. conductivity malleability hardness boiling point 2.2 Physical Properties Assessment Questions 3. In choosing a material for use as a wire to carry electric current, which physical property would be most important? a. b. c. d. conductivity malleability hardness boiling point ANS: A 2.2 Physical Properties Assessment Questions 4. Which of these statements best describes a physical change in a pure substance? a. The substance changes into one or more new substances. b. Some of the properties of the substance change, but the material remains the same. c. The properties of the material do not change, and the material remains the same. d. The substance is separated into two or more simpler substances. 2.2 Physical Properties Assessment Questions 4. Which of these statements best describes a physical change in a pure substance? a. The substance changes into one or more new substances. b. Some of the properties of the substance change, but the material remains the same. c. The properties of the material do not change, and the material remains the same. d. The substance is separated into two or more simpler substances. ANS: B 2.2 Physical Properties Assessment Questions 1. The process of filtration uses the difference in boiling points of substances to separate a mixture. True False 2.2 Physical Properties Assessment Questions 1. The process of filtration uses the difference in boiling points of substances to separate a mixture. True False ANS: F, distillation