Clinical Research Document Checklist
advertisement
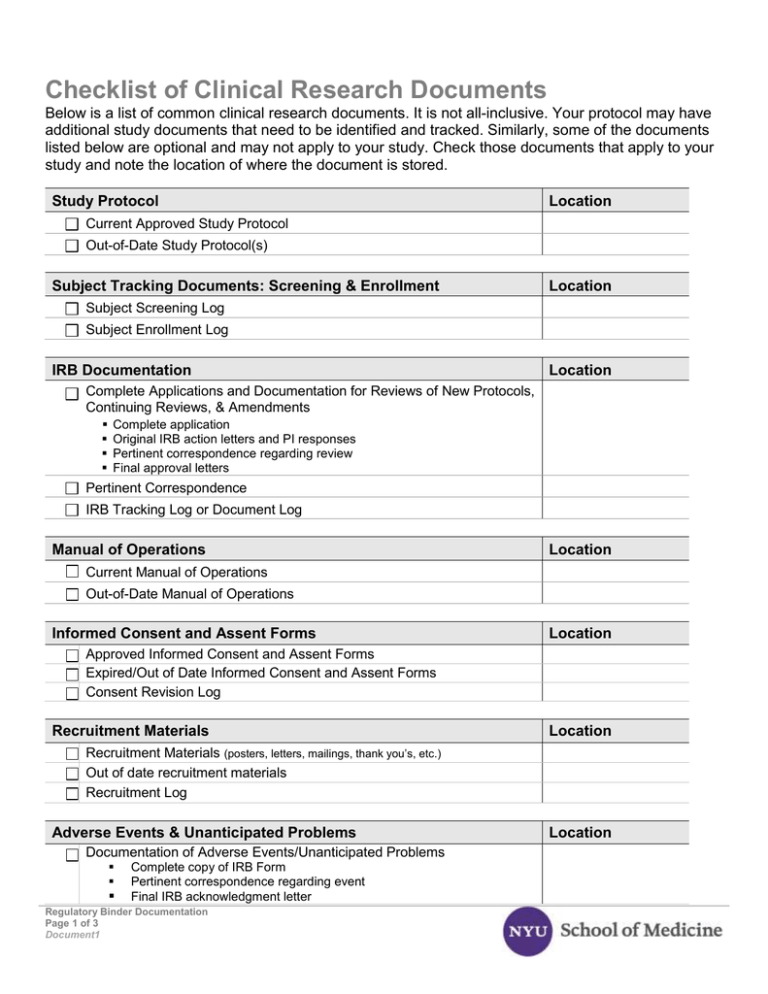
Checklist of Clinical Research Documents Below is a list of common clinical research documents. It is not all-inclusive. Your protocol may have additional study documents that need to be identified and tracked. Similarly, some of the documents listed below are optional and may not apply to your study. Check those documents that apply to your study and note the location of where the document is stored. Study Protocol Location Current Approved Study Protocol Out-of-Date Study Protocol(s) Subject Tracking Documents: Screening & Enrollment Location Subject Screening Log Subject Enrollment Log IRB Documentation Location Complete Applications and Documentation for Reviews of New Protocols, Continuing Reviews, & Amendments Complete application Original IRB action letters and PI responses Pertinent correspondence regarding review Final approval letters Pertinent Correspondence IRB Tracking Log or Document Log Manual of Operations Location Current Manual of Operations Out-of-Date Manual of Operations Informed Consent and Assent Forms Location Approved Informed Consent and Assent Forms Expired/Out of Date Informed Consent and Assent Forms Consent Revision Log Recruitment Materials Location Recruitment Materials (posters, letters, mailings, thank you’s, etc.) Out of date recruitment materials Recruitment Log Adverse Events & Unanticipated Problems Documentation of Adverse Events/Unanticipated Problems Complete copy of IRB Form Pertinent correspondence regarding event Final IRB acknowledgment letter Regulatory Binder Documentation Page 1 of 3 Document1 Location Protocol Deviations and Exceptions Location Documentation of Significant Protocol Deviations/Exceptions Complete copy of IRB Form Pertinent correspondence regarding event Final IRB acknowledgment letter Documentation of Minor Protocol Deviations/Exceptions Minor deviation documentation (memo-to-file/Deviation log) Case Report Forms & Study & Subject Documents Current Case Report Forms (CRFs) (blank) Out of Date CRFs List of Source Documents Study and Subject Documents (blank copy of data collection tools, etc.) Location Reports: DSMB and Monitoring Reports Location Monitoring Reports and DSMB Reports Monitoring Log Documentation of Sponsor Visits (evaluation, initiation, periodic monitoring, closeout) Regulatory Documentation FDA Form 1572 FDA Form 1571 IDE Statement of Investigator’s Commitment Annual Reports Financial Disclosure Form and Information Drug/Device Accountability Logs Sample Label for Investigational Product IRB Composition Location Study Staff Logs and CVs Location Roles & Responsibilities Log Staff Signature Log Research Staff CVs and Qualifications Training Documentation Log Laboratory Documentation Normal Value/Range(s) Certification/Accreditation for Facilities Lab Director’s CV CLIA Certification Certification of Analysis Regulatory Binder Documentation Page 2 of 3 Document1 Location Investigational Brochures Location Current Investigational Brochures Out of Date Investigational Brochures Investigational Brochure updates IRB acknowledgment of new Investigational Brochures/updates Study Drug/Device Shipping records (dates, batch numbers, methods) Accountability logs Randomization code Location Scientific Review Documentation of Scientific Review Location Copies of scientific reviewer worksheets Copies of pertinent correspondence Documentation of department/division approval Subject Case Histories Location Signed informed consent/assent forms Signed, dated and completed case report forms Documentation of CRF corrections Source documents Investigator Agreement Investigator Agreement Confidentiality agreement b/w investigator & sponsor Insurance or indemnification letter Location Miscellaneous Equipment and Supplies (receipt, calibration and maintenance logs) Financial agreements (budget contracts) Location Regulatory Binder Documentation Page 3 of 3 Document1



