2004_jovani&blas_jeb 17 294-301.doc
advertisement

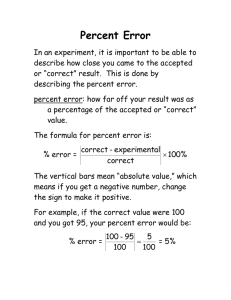
doi: 10.1111/j.1420-9101.2003.00680.x Adaptive allocation of stress-induced deformities on bird feathers R. JO VANI * & J. B LA S * *Department of Applied Biology, Estacio´n Biolo´gica de Doñana, Consejo Superior de Investigaciones Cientı´ficas, Sevilla, Spain Department of Biology, University of Saskatchewan, Saskatoon, Saskatchewan, Canada Keywords: Abstract birds; Ciconia ciconia; fault bar allocation hypothesis; feathers; flight; stress. Physiological stress during ontogeny is known to cause abnormalities in keratin structures of vertebrates, but little is known about if and how organisms have evolved mechanisms to reduce the negative effects of these abnormalities. Stress experienced during avian feather growth is known to lead to the formation of fault bars, and thereby to the weakening of feathers because of shortage and slimming of barbules. Here we propose and test a new hypothesis (the ‘fault bar allocation hypothesis’) according to which birds should have evolved adaptive strategies to counteract this evolutionary pressure. In particular, we predicted and tested the idea that in flying birds, natural selection should have selected for mechanisms to reduce fault bar load on feathers with high strength requirements during flight. Data on the growth of feathers of nestling white storks (Ciconia ciconia) revealed a consistent allocation of more, and more intense, fault bars in innermost than in outermost wing feathers as predicted by our hypothesis. Moreover, the same pattern emerged from feathers of adult storks. We discuss the generality of our results, and suggest avenues for further investigations in this area. Introduction Physiological stress caused by many unpredictable phenomena such as storms, droughts, famine or predation episodes can lower the fitness of organisms. Not surprisingly, coping with stressors has been a constant driving force of evolution (Futuyma, 1998; Buchanan, 2000; Grishkan et al., 2003). In vertebrates, different stressors are mirrored on growth abnormalities in keratin structures such as beaks, claws, feathers, hairs, hoofs, horns or nails. This has been known in medicine for a long time in structures such as nails, where these growth abnormalities have been traditionally associated with episodes of recent stress produced by disease (Han et al., 2000; Ciastko, 2002), or intrusive medical treatments (Deliliers & Monni, 2001; Vassallo et al., 2001). However, although being the symptoms of some kind of stress, they probably have a low, if any, effect per se on the fitness of individuals. Correspondence: Roger Jovani, Department of Applied Biology, Estació n Bioló gica de Doñ ana, Consejo Superior de Investigaciones Cientı́ficas, Avda. Ma Luisa s/n, E-41013 Sevilla, Spain. Tel.: 34 954 232340; fax: 34 954 621125; e-mail: jovani@ebd.csic.es 294 Fault bars in birds are common and long known feather growth abnormalities (Riddle, 1908). Feathers grow from the follicle collar by the accumulation of keratin produced internally by keratinocytes prior to cell death. Fault bars are the result of a variable time lag on the deposition of keratin (Murphy et al., 1989; Prum & Williamson, 2001), producing absence and slimming of barbules, and hence, appearing as narrow (<2 mm) translucent bands across the feather vane almost perpendicular to the feather axis. Consequently, fault bars weaken the feather, increasing the probability of partial or even complete feather breakage along the fault bar line (Slagsvold, 1982; Newton, 1986; Machmer et al., 1992). Like the nails of humans, fault bars have been related to an array of stressors such as malnutrition (Slagsvold, 1982; Newton, 1986), handling (King & Murphy, 1984; Murphy et al., 1988), or mechanical stress experienced during feather formation (Murphy et al., 1989; Negro et al., 1994), leading veterinaries to use fault bars as a symptom of stress and disease suffered by captive and wild birds (Ritchie et al., 1994). However, proximal mechanisms and stressors that produce fault bars are far from being completely resolved, and need of further study. J. EV OL. BIOL. 17 (2004) 294–301 ª 2004 BLACKWELL PUBLISHING LTD Fault bar allocation in birds Contrary to other kind of abnormalities in keratin structures, fault bars could seriously reduce individual birds’ fitness because of their association with flight requirements of birds. Once feathers reach their final length (a matter of weeks or months) they will remain attached to the body of the bird until the next moult (a matter of months or years). Although lost feathers are immediately replaced, broken feathers will be replaced only in the next moult. Thus, feather damage resulting from fault bars may seriously reduce wing surface area for long periods of time. All of this is relevant for bird fitness because wing load (wing area/body weight) is crucial for bird flight performance (Pennycuick, 1989), birds being forced to reduce their weight during natural, i.e. moult, or experimental reductions of wing area (Swaddle & Witter, 1997; Lind & Jakobson, 2001; Senar et al., 2002). Moreover, experimental reduction of wing area is known to increase energetic demands resulting in direct fitness costs as reflected in lowered reproductive success (Mauck & Grubb, 1995; Velando, 2002). Furthermore, fault bars are taxonomically ubiquitous among wing and body feathers (Duerden, 1909; Murphy et al., 1988; Machmer et al., 1992; Negro et al., 1994), suggesting a role for fault bars as an important handicap through the evolutionary history of birds. However, it is surprising that the evolution of mechanisms directed to counteract the negative effects of fault bars in birds remains unclear. Here, we propose and test the hypothesis (‘fault bar allocation hypothesis’) that birds should have evolved adaptive strategies for reducing the costs of fault bars. There is previous evidence for fault bars being more frequent on tail and body feathers than on flight wing feathers of birds (e.g. Slagsvold, 1982; Machmer et al., 1992; Bortolotti et al., 2002), suggesting a differential allocation of fault bars in feathers depending on their importance for flying. However, most of the evidence is circumstantial (e.g. Murphy et al., 1988), and all of the studies up to date have been carried out at the population level rather than at the individual level, and often from feathers grown during different years, being therefore unsuitable for a test of the ‘fault bar allocation hypothesis’. Hence, using individual data on white storks (Ciconia ciconia), we tested the prediction that individuals of flying species should allocate fault bars according to feather strength requirements during flight. As feather requirements are higher for outermost wing feathers (Corning & Biewener, 1998) we predicted that these feathers would have less, and slighter fault bars than the innermost wing feathers. sized (c. 3500 g), long-distance migratory bird (Cramp & Simmons, 1977), and is an appropriate model for this study because fault bars are easily measured even in the smallest wing feathers, and because their occurrence could be potentially shaped by natural selection given the imminence of long-distance migration shortly after feather formation. Feathers from nestling and adult white storks were studied during the breeding season of 2003 at the ‘Dehesa de Abajo’ site (Puebla del Rı́o, Sevilla, SW Spain; 37°13¢N 6°09¢W), where c. 250 pairs of the species nest colonially on wild olive trees. Analyses of nestlings Fifty nestlings aged between 14 and 57 days were included in the analyses. From each nestling, we measured the vane portion emerging from the feather sheath (i.e. where fault bars can be detected) of three feathers from each one of the main flight feather groups of the left wing: primaries (hereafter P), primary coverts (PC), secondaries (S), secondary coverts (SC), scapulars (Sc) and scapular coverts (ScC, Fig. 1). In this way, we examined a representative sample of each group of feathers while minimizing disturbance to the nestlings and the rest of the colony. Even so, we were forced to skip recording fault bar occurrence on the last group of feathers (ScC) in 17 occasions because of time constraints. Each feather was carefully checked for the presence of fault bars, categorizing them as light (absence of some barbules producing a visible discontinuity on the structure of the feather), medium (a narrow, i.e. <1 mm, translucent line across the feather), or strong (‡1 mm translucent line across the feather, many times causing breakage of edge barbs of the feather). The distance (to the nearest mm) from each fault bar to the tip of the feather was also recorded. Primaries Methods The study species and population This study was performed on feathers from both nestling and adult white storks (C. ciconia). This species is a large 295 Secondaries Scapulars Fig. 1 Location of the studied feathers within the left wing of a nestling white stork. Feathers inspected in nestlings are indicated in dark grey. Feathers studied in adults are shown in dark and light grey. Nonstudied feathers are indicated in white. J. EVOL. BIOL. 17 (2004) 294–301 ª 2004 BLACKWEL L P UBLISHIN G LTD 296 R. J O VA N I AND J . B L A S 200 Feather type PC5 S10 SC10 Sc3 ScC3 175 Feather length (mm) 150 125 100 75 50 25 0 0 25 50 75 100 125 150 175 200 P5 length (mm) Fig. 2 Length of the exposed portion of growing feathers from 50 nestlings relative to the length of exposed fifth primary feather. Only the central feather of each type is plotted for clarity. Lines represent best fitting equations. See text for further details. proportion of fault bars in feathers was analysed with a binomial distribution of errors and a logit link function, and the number of fault bars per feather with a Poisson distribution of errors and a log link function. Feather identity (e.g. third primary, second scapular) was fitted into the model as a factor. Moreover, we used Binomial tests and Wilcoxon matched-pairs signed-rank tests for analysing how consistent those general (i.e. population level) results found in GLMM analyses were at the individual level. These analyses were conservative since we also included the 17 nestlings for which we did not check fault bars on the ScC (the exclusion of these nestlings from the analyses did not change any of the results). For clarity, we have analysed the occurrence of all fault bars together (light, medium and strong), and strong fault bars separately because of their potential difference in functional importance. However, light and medium fault bars separately showed very similar patterns. Comparison between adults and nestlings To properly assess whether individuals differentially allocate fault bars among feather types, one must compare portions of feathers that have grown at the same time at different parts of the given bird. Thus, we first studied the growth of each feather relative to the others on the 50 nestlings (Fig. 2). Secondaries were the latest feathers to be grown whereas PC and SC were the first to reach their final length (Fig. 2). In this way, the five youngest nestlings on which the vane of S had not started to emerge from the sheaths were excluded from the analysis. Moreover, we selected the first, second or third order equation best fitting to the data of the relationship between P5 (i.e. the longest P) and each of the other 17 feather types (examples in Fig. 2). With these 17 equations we calculated the length of each feather when S start growing (left arrow in Fig. 2), and when PC and SC achieve their final length (right arrow in Fig. 2). We did it in a conservative way, assuming that some PC-SC could achieve their final length when P5 is 132 mm long. Thus, from the remaining 45 nestlings, we discarded those feather portions that were growing at times when other feathers either had not initiated or had already finished their growth (i.e. proximal feather parts that were still growing when PC and SC had stopped growing; and tip zones of those feathers that were growing before the vanes of S started to emerge from their sheaths). As the occurrence of fault bars in a particular feather is potentially related to the occurrence of fault bars in the other feathers of an individual nestling, we considered feathers as nonindependent units in the analyses. Therefore, nestling identity was used as a random factor in generalized linear mixed models (GLMMs), using GLIMMIX macro of SAS to control for the different occurrence of fault bars among nestlings (Littell et al., 1996). The The study of fault bar allocation in adults involves technical and ethical considerations because storks moult during the breeding season (Hall et al., 1987), and the asynchronous growth of adult feathers (Hall et al., 1987) precludes any study of such detail as that conducted in nestlings. Fortunately, freshly moulted adult feathers accumulate on the ground, and we seized this opportunity by collecting more than 900 adult feathers during our incursions into the colony in June 2003. Feathers were later classified in the laboratory according to a reference feather collection, and feathers that were clearly different from those studied in nestlings were excluded, that is, the innermost P (shorter, and less emarginated, and thus with a probable different flight function), the innermost S (weaker than the rest of S), and the first Sc (noticeably smaller than the rest of Sc; see Fig. 1). Hence, 753 adult feathers were available for the study of the number and intensity of fault bars (see Fig. 5 for sample sizes of different feather types). White stork nestlings have been banded with plastic rings at the study site and many other localities in Spain (c. 19 000 nestlings) from 1986 onwards. In particular, during the breeding season that preceded this study, we ringed 217 nestlings at the study colony (c. 95% of the fledged storks), and c. 400 nestlings were banded in nearby stork nests. In this way, based on our periodic surveys of the study site searching for banded storks from 1 month before we started to collect the adult feathers, we were able to gather strong evidence supporting that the collected feathers were from adult storks, as not a single first year stork was found in the colony from a total of 53 storks that we read the plastic bands of. As we sampled nestlings at different growing stages, many had small feathers, precluding a reliable comparison with fully grown feathers of adults. Thus, we J. EV OL. BIOL. 17 (2004) 294–301 ª 2004 BLACKWELL PUBLISHING LTD Fault bar allocation in birds 10 20 9 8 SC-Sc-ScC 6 5 4 3 2 1 ScC3 ScC4 ScC3 ScC4 Sc3 Sc3 Sc4 Sc2 Sc2 ScC2 SC12 SC12 ScC2 SC10 SC10 Sc4 S12 SC8 S12 SC8 S8 S10 14 PC7 Strong PC5 P7 5 P5 0 4 All fault bars 7 3 2 0 P-PC-S 1 SC-Sc-ScC S10 S8 PC7 PC5 PC3 P7 P5 0 P3 Innermost wing feathers were more prone to have at least one fault bar than outermost wing feathers (GLMM, F17,697 ¼ 15.56, P < 0.0001; Fig. 3). The proportion of feathers with fault bars was between 4.4 and 22.2% on P-PC-S, but it was much higher in SC (between 53.3 and 62.2%) and especially on Sc-ScC (57.8–92.9%; Fig. 3). Two nestlings had no fault bars along the wing, and 26 had fault bars both on P-PC-S, and on SC-Sc-ScC. All 17 nestlings having only fault bars on one of these two groups of feathers had fault bars on inner, instead of outer wing feathers (Binomial test P < 0.0001; Fig. 4 insert). The number of fault bars also increased towards inner wing feathers (GLMM, F17,697 ¼ 32.48, P < 0.0001; Fig. 4). P-PC-S that had fault bars normally had a single one and never more than three fault bars. However, two fault bars were common for SC and up to four or five fault bars were common for Sc and ScC, respectively. Feather type Fig. 4 Number of fault bars of any kind (upper box), and only strong fault bars (lower box) in nestlings. Lines represent fault bars along the wing of individual nestlings. Median (quartiles) are indicated by circles. Inserts resume major graphs by showing the number of fault bars within nestlings on feather types primaries-primary covertssecondaries vs. secondary coverts-scapulars-scapular coverts (ScC). Broken lines represent those nestlings on which we did not recorded the occurrence of fault bars on the ScC. Moreover, almost all nestlings had more fault bars on inner than on outer wing feathers (Fig. 4; Table 1). Strong fault bars 100 Percentage feathers with fault bars 0 P-PC-S 7 PC3 Fault bar distribution on nestling wings 40 All 11 P3 Results 12 Number of fault bars selected those 20 oldest nestlings from the original data set, which had at least 100 mm of exposed P5. This ensures that most fault bars occurring on nestling feathers were recorded, as fault bars typically accumulate at the tip of the feathers (R. Jovani & J. Blas, unpublished data). For statistical analyses we used generalized lineal models (GLM) with the same distributions of errors and link functions as described above. In this case, we used the feather group (e.g. primaries, secondaries), the age (nestling or adult), and their interaction as the independent variables. 297 90 All Strong 80 70 60 50 40 30 20 10 ScC4 ScC3 Sc4 ScC2 Sc3 Sc2 SC12 SC10 S12 SC8 S8 S10 PC7 PC5 P7 45 45 45 45 45 28 28 28 PC3 45 45 45 45 45 45 45 45 45 45 P5 P3 0 Feather type Fig. 3 Proportion of nestling feathers with fault bars. The number of each type of feather analysed is indicated. P-PC-S were almost free from strong fault bars (Fig. 3). However, the proportion of feathers with some strong fault bar increased from SC to ScC (GLMM, F17,697 ¼ 9.97, P < 0.0001; Fig. 3). Seventeen nestlings lacked strong fault bars in any feather, and two had fault bars both on P-PC-S, and SC-Sc-ScC. Twenty-five of those 26 nestlings having fault bars only on P-PC-S, or only on SC-Sc-ScC, had fault bars on inner but not on outer wing feathers (Binomial test, P < 0.0001; Fig. 4 insert). Similarly, the number of strong fault bars was unequally distributed among the wing feathers of nestlings (GLMM, F17,697 ¼ 25.99, P < 0.0001), being higher in inner wing feathers (Fig. 4). P-PC-S had as much as one single strong fault bar, SC two, Sc three, and ScC reached a maximum of five fault bars and median values up to one strong fault bar. Moreover, almost all nestlings had equal or higher numbers of strong fault bars on inner J. EVOL. BIOL. 17 (2004) 294–301 ª 2004 BLACKWEL L P UBLISHIN G LTD 298 R. J O VA N I AND J . B L A S Table 1. Wilcoxon matched-pairs signed-rank tests for examining the difference on the number of fault bars occurring on feather types P-PC-S vs. SC-Sc-ScC (see Fig. 1 for the position of the feathers on the wing) on the 45 nestlings studied. Data refer to the number of nestlings in each class. 100 90 Chicks All Strong 80 70 60 Type of fault bars P, PC, S < SC, Sc, ScC P, PC, S ¼ SC, Sc, ScC P, PC, S > SC, Sc, ScC Wilcoxon test Strong 42 3 0 Z ¼ )5.647, P < 0.0001 27 17 1 Z ¼ )4.530, P < 0.0001 P, primaries; PC, primary coverts; S, secondaries; SC, secondary coverts; Sc, scapulars; ScC, scapular coverts. vs. outer feathers, with only one nestling showing the opposite pattern (Table 1; Fig. 4 insert). Percentage feathers with fault bars 50 All 40 30 20 10 0 60 60 60 60 60 24 P PC S SC Sc ScC 100 90 Adults All Strong 80 70 60 Comparison between adults and nestlings 50 All fault bars 40 As a whole, the proportion of feathers with fault bars was three times higher in nestlings (43.2%, n ¼ 324) than in adults (12.9%, n ¼ 753; v32 ¼ 754.76, P < 0.0001), and this pattern holds true even when controlling for feather type (GLM, feather type v25 ¼ 277.72, P < 0.0001; age v21 ¼ 99.27, P < 0.0001). Although fault bars predominated on inner wing feathers in both nestlings and adults, a clear age-related difference occurred; whereas in the nestlings almost all the feathers with fault bars were SC-Sc-ScC, in the adults fault bars mainly occurred on Sc-ScC, but not in SC (GLM, feather type v25 ¼ 277.72, P < 0.0001; age v21 ¼ 99.27, P < 0.0001; interaction feather type · age: v25 ¼ 41.07, P < 0.0001; Fig. 5). As a whole, nestlings showed a higher number of fault bars (mean ¼ 2.80) than adults (mean ¼ 2.44; Mann– Whitney U324,753 ¼ 86599.50, P < 0.0001), and this hold when statistically controlling for feather type (GLM, feather type v25 ¼ 1054.43, P < 0.0001; age v12 ¼ 117.04, P < 0.0001). Inner wing feathers harboured more fault bars than outer wing feathers both in nestlings and adults (Fig. 5). However, this increase started on SC in nestlings and in Sc in adults (GLM, feather type v25 ¼ 1054.43, P < 0.0001; age: v21 ¼ 117.04, P < 0.0001; age · feather type v25 ¼ 151.77, P < 0.0001). Moreover, the maximum numbers of fault bars also increased towards inner wing feathers in the nestlings (Spearman correlation with the two maximum values of each feather type rs ¼ 0.812, n ¼ 12, P < 0.01), but not in the adults (rs ¼ 0.429, n ¼ 12, n.s.; Fig. 6). Strong fault bars The proportion of feathers with strong fault bars was six times higher in nestlings (9.7%, n ¼ 324) than in adults 30 20 10 0 124 68 256 205 61 39 P PC S SC Sc ScC Feather type Fig. 5 Proportion of nestling and adult feathers with at least one fault bar. The number of analysed feathers of each type is indicated. (1.6%, n ¼ 753; v32 ¼ 1279.48, P < 0.0001), and the same was found when controlling for feather type in a GLM analysis (feather type v52 ¼ 134.79, P < 0.0001; age v21 ¼ 28.32, P < 0.0001). As for the total proportion of fault bars, the interaction between age and feather type was also significant in the multivariable analysis (GLM, feather type v25 ¼ 134.79, P < 0.0001; age v12 ¼ 28.32, P < 0.0001; feather type · age v52 ¼ 21.63, P < 0.01), indicating that while in nestlings the proportion of strong fault bars gradually increased from S up to ScC, this was displaced one position towards inner wing feathers in adults, only increasing from SC to Sc, but not from S to SC (Fig. 5). Nestlings showed a higher number of strong fault bars (mean ¼ 0.188) than adults (mean 0.021, Mann– Whitney U324,753 ¼ 112052.00, P < 0.0001), and this hold when statistically controlling for feather type (GLM, feather type v25 ¼ 256.23, P < 0.0001; age v21 ¼ 46.10, P < 0.0001). Inner wing feathers had more numerous strong fault bars both in nestlings and adults (Fig. 6). In nestlings, strong fault bars showed a clear increase towards ScC, but in adults Sc was the feather type with more strong fault J. EV OL. BIOL. 17 (2004) 294–301 ª 2004 BLACKWELL PUBLISHING LTD Number of fault bars Fault bar allocation in birds 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 Chicks 50 59 P 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 All Strong 54 60 PC Adults 117 124 P 52 60 S 21 57 7 SC 50 Sc 0 4 ScC All Strong 61 69 PC 239 S 256 192 202 SC 32 53 Sc 15 38 ScC Feather type Fig. 6 Number of fault bars in nestling and adult feathers. Circle size represents the number of feathers from one to 16. The number of feathers with zero fault bars is also indicated. bars (GLM, feather type v25 ¼ 256.23, P < 0.0001; age v21 ¼ 46.10, P < 0.0001; age · feather type v25 ¼ 28.45, P < 0.0001). Discussion Our results demonstrate that white stork nestlings confronted with natural stressors consistently allocated fault bars on the different wing feathers in a nonrandom manner. This gives support to our hypothesis of adaptive allocation of fault bars, because feathers with important flight function were those showing a lower proportion and number of both total and strong fault bars. Moreover, despite the intrinsic methodological difficulties in the study of fault bar allocation in adults, the same pattern was found on adult feathers, suggesting an adaptive fault bar allocation strategy in white storks throughout their lives. Furthermore, the lower number of fault bars in adults paralleled a movement of the first feather type with high fault bars values towards inner wing feathers (Sc) in relation to nestlings (SC), suggesting that fault bar allocation occurs in an hierarchical manner. This also proves that fault bar occurrence is not 299 dependent of feather strength per se, but rather on feather location. Previous studies have also found different occurrence of fault bars on different feather groups (Machmer et al., 1992), but to our knowledge, this is the first clear demonstration of the occurrence of fault bar allocation because feather zones growing at the same time in the same individuals were compared. Consistent with previous information (e.g. Slagsvold, 1982), fault bars were more common and stronger in nestlings than in adults. This could be because nestlings are forced to grow all feathers in a short time period while the moult in the adults of the studied species is much more flexible in timing, and feathers grow asynchronously. It is worth noting, however, that while extreme numbers of fault bars in nestlings were only found on inner wing feathers, even some outermost adult feathers showed a high frequency of fault bars. Because of fault bar allocation, this finding suggests that some adults could be suffering even higher physiological stress than nestlings. Fault bars have been used as an index of ‘stress’, ‘quality’, or ‘body condition’ in an array of studies in animal ecology (Bortolotti et al., 2002), conservation biology (Sodhi, 2002), foraging ecology (Waite, 1990), or sex allocation (Slagsvold, 1982). Fault bars occurring on different feather types are typically added to extract a ‘fault bar index’ (Bortolotti et al., 2002), or only fault bars occurring on one feather type are recorded (Sodhi, 2002). Because fault bar allocation is not random, information contained in fault bars occurring on different feathers should not be equivalent, but rather complementary. Therefore, we suggest that the best way of extracting information from fault bars could be first exploring fault bar data, and then studying separately fault bar occurrence on different feather groups, or weighting fault bar occurrence on different feathers proportionally to their rarity on the study species. Our results with storks together with previous evidence (Murphy et al., 1988; Machmer et al., 1992) suggest that fault bar allocation could be a common strategy of many flying bird species. However, the ‘fault bar allocation hypothesis’ predicts that fault bar allocation might be a common strategy in species with high flight requirements, but may be absent on nonflying species. Ostriches (Sthrutio camelus) could provide some insight on this point. Thanks to the economic importance of ostrich feathers (e.g. dusters, decorative purposes), farmers have studied the occurrence of fault bars in ostrich feathers for a long time (Duerden, 1909; Deeming, 1999). Fault bars are common both in wild and farmed ostriches, and they are equally abundant on wing and body feathers (Duerden, 1909). This supports our fault bar allocation hypothesis, because nonflying birds such as ostriches seem to show a similar propensity of fault bars on flight and body feathers. Moreover, although the occurrence of fault bars has also been related to many external stressors in ostriches (e.g. bad J. EVOL. BIOL. 17 (2004) 294–301 ª 2004 BLACKWEL L P UBLISHIN G LTD 300 R. J O VA N I AND J . B L A S weather, inappropriate handling, crowding), some genetic variability in the propensity to develop fault bars has been detected, and directed captive breeding programmes have brought some improvements in the quality of feathers (Petitte & Davis, 1999). Thus, a prerequisite of our hypothesis, i.e. that there is genetic variability in fault bar propensity available for natural selection to operate on, is confirmed by ostrich information. The potential of fault bars in biological research is still undervalued. For instance, like tree-ring data (e.g. Swetnam & Lynch, 1993; Barber et al., 2000), the study of fault bars from museum bird collections could permit the reconstruction of the ‘stress history’ of birds during the last centuries. However, this promising future will benefit of further work on the proximal mechanisms producing fault bars (Murphy et al., 1988), the covariation between fault bars and fitness (e.g. Bortolotti et al., 2002), and further tests of the ‘fault bar allocation hypothesis’. Acknowledgments We are grateful to José Luis Tella for his support. Joaquı́n Lamas, Judit Smits, Gary Bortolotti, José Luis Tella, Martina Carrete, Raquel Baos, Isa Luque, Mariola Martı́n, MaCarmen Roque, Fatima Leó n, Ernesto Fedriani and especially Juan Manuel Terrero were of great help on the field. This work improved from discussions and manuscript revisions from Gary Bortolotti, José Luis Tella, David Serrano, Juha Merilä , Carlos Rodriguez, Esperanza Ursú a and Anna Perera. Funds were provided by the Project B0S2002-00857 of Ministerio de Ciencia y Tecnologı́a y Fondos Feder, Telefó nica Mó viles S.A. and Junta de Andalucı́a. JB was supported from a postdoctoral grant from the Spanish Ministerio de Educació n, Cultura y Deporte. References Barber, V.A., Juday, G.P. & Finney, B.P. 2000. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature 405: 668–673. Bortolotti, G.R., Dawson, R.D. & Murza, G.L. 2002. Stress during feather development predicts fitness potential. J. Anim. Ecol. 71: 333–342. Buchanan, C.L. 2000. Stress and the evolution of conditiondependent signals. Trends Ecol. Evol. 15: 156–160. Ciastko, A.R. 2002. Onychomadsis and Kawasaki disease. Can. Med. Assoc. J. 166: 1069. Corning, W.A. & Biewener, A.A. 1998. In vivo strains in pigeon flight feather shafts: implications for structural design. J. Exp. Biol. 201: 3057–3066. Cramp, S. & Simmons, K.E.L. 1977. The Birds of the Western Paleartic, Vol 1. Oxford University Press, Oxford. Deeming, D.C. 1999. The Ostrich: Biology, Production & Health. CAB International, UK. Deliliers, G.L. & Monni, P. 2001. Nail transverse white bands induced by antileukemic chemotherapy. Haematologica 86: 333. Duerden, J.E. 1909. Experiments with ostriches – X. How the bars in ostrich feathers are produced. Agric. J. Cape Good Hope 35: 474–484. Futuyma, D.J. 1998. Evolutionary Biology. Sinauer, MA, USA. Grishkan, I., Korol, A.B., Nevo, E. & Wasser, S.P. 2003. Ecological stress and sex evolution in soil microfungi. Proc. R. Soc. Lond. B 270: 13–18. Hall, M.R., Gwinner, E., Bloesch, M. 1987. Annual cycles in moult, body mass, luteinizing hormone, prolactin and gonadal steroids during the development of sexual maturity in the White Stork (Ciconia ciconia). J. Zool. 211: 467–486. Han, R.K., Sinclair, B., Newman, A., Silverman, E.D., Taylor, G.W., Walsh, P. & McCrindle, B.W. 2000. Recognition and management of Kawasaki disease. Can. Med. Ass. J. 162: 807–812. King, J.R. & Murphy, M.E. 1984. Fault bars in the feathers of White-crowned sparrows: dietary deficiency or stress of captivity and handling? Auk 101: 168–169. Lind, J. & Jakobson, S. 2001. Body building and concurrent mass loss: flight adaptations in tree sparrows. Proc. R. Soc. Lond. B. 268: 1915–1919. Littell, R.C., Milliken, G.A., Stroup, W.W. & Wolfinger, R.S. 1996. SAS System for Mixed Models. SAS Institute Inc, Cary, NC, USA. Machmer, M.M., Esselink, H., Steeger, C. & Ydenberg, R.C. 1992. The occurrence of fault bars in the plumage of nestling ospreys. Ardea 80: 261–272. Mauck, R.A. & Grubb, T.C. Jr. 1995. Petrel parents shunt all experimentally increased reproductive costs to their offspring. Anim. Beh. 49: 999–1008. Murphy, M.E., King, J.R. & Lu, J. 1988. Malnutrition during the postnuptial molt of White-crowned sparrows: feather growth and quality. Can. J. Zool. 66: 1403–1413. Murphy, M.E., Miller, B.T. & King, J.R. 1989. A structural comparison of fault bars with feather defects known to be nutritional induced. Can. J. Zool. 67: 1311–1317. Negro, J.J., Bildstein, K.L. & Bird, D.M. 1994. Effects of food deprivation and handling stress on fault-bar formation in nestling American kestrels. Ardea 82: 263–267. Newton, I. 1986. The Sparrowhawk. Poyser, Calton. Pennycuick, C.J. 1989. Bird Flight Performance: A Practical Calculation Manual. Oxford University Press, Oxford. Petitte, J.N. & Davis, G. 1999. Breeding and genetics. In: The Ostrich: Biology, Production and Health (D. C. Deeming, ed.), pp. 275–292. CAB International, UK. Prum, R.O. & Williamson, S. 2001. Theory of the growth and evolution of feather shape. J. Exp. Zool. 291: 30–57. Riddle, O. 1908. The genesis of fault bars in feathers and the cause of alternation of light and dark fundamental bars. Biol. Bull. 14: 328–371. Ritchie, B.W., Harrison, G.J. & Harrison, L.R. 1994. Avian Medicine: Principles and Application. Wingers, FL, USA. Senar, J.C., Domè nech, J. & Uribe, F. 2002. Great tits (Parus major) reduce body mass in response to wing area reduction: a field experiment. Beh. Ecol. 13: 725–727. Slagsvold, T. 1982. Sex, size, and natural selection in the Hooded crow Corvus corone cornix. Ornis Scand. 13: 165–175. Sodhi, N.S. 2002. A comparison of bird communities of two fragmented and two continuous southeast Asian rainforests. Biod. Cons. 11: 1105–1119. Swaddle, J.P. & Witter, M.S. 1997. Food availability and primary feather molt in European starlings, Sturnus vulgaris. Can. J. Zool. 75: 948–953. J. EV OL. BIOL. 17 (2004) 294–301 ª 2004 BLACKWELL PUBLISHING LTD Fault bar allocation in birds Swetnam, T.W. & Lynch, A.M. 1993. Multicentury, regionalscale patterns of western spruce budworm outbreaks. Ecol. Monogr. 63: 399–424. Vassallo, C., Brazzelli, V., Ardigò , M. & Borroni, G. 2001. Nail changes in onco-hematologic patients. Haematologica 86, 334– 336. Velando, A. 2002. Experimental manipulation of maternal effort produces differential effects in sons and daughters: implications for adaptive sex ratios in the blue-footed booby. Beh. Ecol. 13: 443–449. 301 Waite, T.A. 1990. Effects of catching supplemental food on induced feather regeneration in wintering Gray Jays Perisoreus canadensis: a ptiochronology study. Ornis Scand. 21: 122–128. Recieved 17 September 2003; revised 4 November 2003; accepted 4 November 2003 J. EVOL. BIOL. 17 (2004) 294–301 ª 2004 BLACKWEL L P UBLISHIN G LTD Copyright of Journal of Evolutionary Biology is the property of Blackwell Publishing Limited and its content may not be copied or emailed to multiple sites or posted to a listseiv without the copyright holder’s express written permission. However, users may print, download, or email articles for individual use.