jovani_et_al_j_zool_2010.doc
advertisement

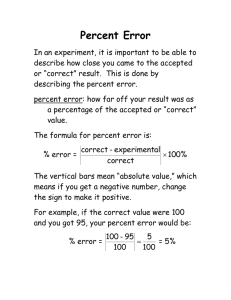
Journal Fault bars and the risk of feather damage in cranes R. Jovani1,2, J. Blas1, M. J. Stoffel3, L. E. Bortolotti3 & G. R. Bortolotti3 1 Department of Conservation Biology, Estación Biológica de Doñana, CSIC, Sevilla, Spain 2 UFZ, Department of Ecological Modelling, Helmholtz Centre for Environmental Research—UFZ, Leipzig, Germany 3 Department of Biology, University of Saskatchewan, Saskatoon, SK, Canada Keywords fault bars; feathers; flight; natural selection; sandhill crane; stress. Correspondence Roger Jovani, Department of Evolutionary Ecology, Estación Biológica de Doñana, CSIC, Avda. Americo Vespuccio s/n 41092, Sevilla, Spain. Email: jovani@ebd.csic.es Abstract Fault bars are translucent areas across feathers grown under stressful conditions. They are ubiquitous across avian species and feather tracts. Because fault bars weaken feather structure and can lead to feather breakage, they may reduce flight performance and lower fitness. Therefore, natural selection might prime mechanisms aimed at reducing the cost of fault bars, penalizing their occurrence in those feathers more relevant for flight. Here, we tested one prediction of this ‘fault bar allocation hypothesis’: that the prevalence, abundance and risk of damage of fault bars change across the wing feathers of a long-distance migrant, the sandhill crane Grus canadensis as a function of the strength requirements of feathers for flight. We analysed 2411 wing feathers with 4676 fault bars from 39 cranes in active migration. Fault bars did not increase feather damage with feather age. The occurrence of fault bars decreased from proximal to distal wing portions, both in flight feathers and in coverts, according to the presumed greater strength requirements of external wing feathers during flight. The occurrence of fault bars was variable when producing low feather damage (o2%) but was consistently low for fault bars with a higher damage probability (42–30%). Altogether, our results suggest that fault bars are common on the feathers of birds even after millions of years of evolution because natural selection seems to penalize birds with particularly harmful fault bars in certain feathers and of a certain magnitude, but is unable to eliminate less harmful fault bars according to their strength and position. Fault bars are common structural abnormalities of feathers produced as they grow (Riddle, 1908). Although the proximal factors generating fault bars are still poorly understood, poor nutrition and stress are commonly invoked (Riddle, 1908; Slagsvold, 1982; King & Murphy, 1984; Machmer et al., 1992). Feathers grow by the accumulation of keratin produced in the follicle collar by keratinocytes. Fault bars are a deficit of keratin that results in the absence or the slimming of some barbules, appearing as narrow (o2 mm) translucent bands across the feather vane, almost perpendicular to the feather rachis (Riddle, 1908; Prum & Williamson, 2001). Because fault bars weaken the feather structure, they increase the probability of partial or even complete feather breakage along the fault bar line (Slagsvold, 1982; Newton, 1986; Machmer et al., 1992; Sarasola & Jovani, 2006; Møller, Erritzøe & Nielsen, 2009). The timing of moult does not change for damaged feathers, and therefore fault bars may decrease the wing area for long periods of time. This might be particularly relevant for species that do not undergo a complete annual moult of flight feathers, as occurs in most large birds (Baker, 1993; Rohwer et al., 2009). Because wing load (i.e. body weight/wing area) is crucial for flight performance (Pennycuick, 1989), and experimental reductions of wing area have increased the energetic demands of birds and reduced their reproductive success (Mauck & Grubb, 1995; Velando, 2002), fault bars could potentially jeopardize individual fitness (Bortolotti, Dawson & Murza, 2002). Jovani & Blas (2004) proposed that fault bars may have imposed a selective pressure on birds to evolve adaptive strategies that minimize the negative consequences of fault bars on fitness. The ‘fault bar allocation hypothesis’ (Jovani & Blas, 2004) was originally proposed in a study of white storks Ciconia ciconia, where the abundance of fault bars along the wing feathers followed a non-random pattern, consistent with specific feather functions and strength requirements for flight. Subsequent evidence in support of the hypothesis emerged from studies in a migratory passerine (Serrano & Jovani, 2005) and a migratory hawk (Sarasola & Jovani, 2006). Here, we studied the occurrence of faults bars on wing feathers of sandhill cranes Grus canadensis to test the ‘fault bar allocation hypothesis’ (Jovani & Blas, 2004) and discuss the results from an evolutionary perspective. Materials and methods Study species and data collection The sandhill crane is a large (3400–4900 g) long-lived (420 years) bird widely distributed in North America (Tacha, Nesbitt & Vohs, 1992). The nominal subspecies breeds in arctic and subarctic North America and eastern Siberia, and performs a long-distance migration to wintering quarters in the south-west USA and north-central Mexico (del Hoyo, Elliot & Sargatal, 1996). Stable isotope analyses suggest that the breeding grounds of our study population span the northern parts of the western Canadian provinces and northward through the Arctic (Hobson et al., 2006). In October 2004, we collected 39 crane carcasses hunted during fall migration in southern Saskatchewan (Canada). The carcasses were salvaged from a commercial hunting camp, and so no animals were harmed or killed for this study. Upon collection, carcasses were transported to Saskatoon and frozen until examination in the laboratory. Some feathers were completely or partially missing (n = 51), or too dirty to be analysed (n = 34), and were excluded from further analyses. The remaining primaries, secondaries, tertials, scapulars and their associated coverts of one wing per bird were carefully inspected for fault bars under constant light conditions by a single observer (M. J. S.) unaware of the hypothesis being tested. Wing flight feathers were classified into five groups: Pext: from the outermost first to the fifth primary, Pint: primaries six to the innermost number 10, S: secondaries, T: tertials and Sc: scapulars. Primaries were divided into two groups because of their distinct morphology and function during flight, the distal ones being more asymmetrical and curving up strongly during flight. This distinction did not apply to coverts and were thus classified as: PC, primary coverts; SC, secondary coverts; TC, tertial coverts and ScC, scapular coverts. A given crane specimen often had different feather generations from different moulting episodes, and we recorded the age of the feathers, that is feathers grown this year, last year or earlier according to feather wear. Fault bars were categorized following Sarasola & Jovani (2006) as light (absence of some barbules producing a visible discontinuity on the structure of the feather), medium (a narrow, i.e. o1 mm, translucent line across the feather) or strong (Z1 mm, translucent line across the feather). We also recorded whether fault bars produced a break in a portion of the vane from its position up to the distal edge of the feather vane (damage), or the complete breakage of the rachis, thus shortening the feather (cut). Statistical analyses Because individual bird behaviour or feather quality would vary among birds, we treated the fault bars of the same bird as non-independent units in the analyses. To do so, we performed a generalized linear mixed model (GLMMs) using the GLIMMIX macro of SAS, where bird identity was treated as a random factor (Littell et al., 1996). Whether the fault bar did (1) or did not (0) produce feather ‘damage’ or ‘cut’ was introduced as the dependant variable, and feather age, fault bar strength and feather type were introduced as independent categorical variables. We used a binomial distribution of errors and a logit link function leading a model equivalent to a logistic regression. The proportion of feathers with fault bars was analysed with a binomial distribution of errors and a logit link function, and the number of fault bars per feather with a Poisson distribution of errors and a log link function. Because the occurrence of fault bars in a particular feather is potentially related to the occurrence of fault bars in the other feathers of an individual, whenever possible, we considered feathers as non-independent units in the analyses. Moreover, unpredictable sources of ‘noise’ could arise on the number of fault bars in feathers of different ages due to a ‘year effect’ (e.g. different environmental conditions among years). To overcome these statistical difficulties, bird identity and the age of the feather were used as random factors in generalized linear mixed models (GLMMs), using GLIMMIX macro of SAS (Littell et al., 1996). Feather group (e.g. secondaries, scapular coverts) was fitted into the model as a factor. For the analysis of the probability of fault bars producing damage, fault bars in growing feathers were considered to be of the same age as those in fresh ones (i.e. 1 year old). When GLMM models did not converge in these analyses, GLM models were used. However, when comparing the occurrence of fault bars in different feathers, the growing feathers were not considered because the number of fault bars could be underestimated. Results Overall, we analysed 2411 feathers: 195 Pext, 193 Pint, 603 S, 114 T, 112 Sc, 383 PC, 592 SC, 114 TC and 105 ScC, from which we recorded a total of 4676 fault bars: 3664 light, 664 medium and 348 strong; see the legend of Fig. 2 for sample sizes according to feather groups. Feather damage was detected for 96 fault bars and 13 feathers were cut by a fault bar. Feather age and fault bars We first tested whether older feathers showed more damage by fault bars as a consequence of a longer exposure to the wear and tear of flight. We did not find, though, any evidence that fault bars increase damage probability with feather age (F3,4634 = 0.69, P = 0.561), and this held true when we controlled for the strength of the fault bar (F3,4632 = 0.76, P = 0.518), feather type (F3,4626 = 0.10, P = 0.958) and both variables together (F3,4625 = 0.01, P = 0.998). Thus, we were confident that our results on fault bar risk of damage were not biased by feather age. Fault bar prevalence In flight feathers, the prevalence of medium and strong fault bars was low (o20%) in secondaries and internal primaries, and slightly higher (20–30%) in tertials, scapulars and external primaries (GLMM, medium: F4,1135 = 5.92, Po 0.0001; strong: F4,1135 = 3.08, P = 0.016). Both light and overall fault bar prevalence were higher in flight feathers, Feather damage Figure 1 Prevalence (a) and abundance (b) of fault bars according to feather group and fault bar magnitude. but particularly so on tertials and scapulars (GLMM, light: F4,1135 = 7.56, Po0.0001; all: F4,1135 = 10.45, Po0.0001). The same patterns of fault bar abundance observed in flight feathers were found in coverts (GLMM, light: F3,1059 = 17.99, Po0.0001; medium: F3,1059 = 8.40, Po0.0001; strong: F3,1059 = 19.99, Po0.0001; all: F3,1059 = 20.97, Po0.0001; Fig. 1a). Thirteen feathers were broken due to fault bars (one light, four medium and eight strong fault bars). Because of this small number of feathers broken by fault bars, we analysed feather breakage and vane damage together (n = 109). As expected, the risk of feather damage on flight feathers differed among fault bar strengths (w22 = 1231.64, Po0.0001). There was a low risk for light fault bars (0.2% of feathers), intermediate for medium (4.0%) and moderate (14.4%) for strong fault bars. A similar pattern occurred in covert feathers (w22 = 122.83, Po0.0001): light (1.1%), medium (8.0%) and strong (17.8%) fault bars. Within a fault bar strength category, the risk of feather breakage was equal across flight feathers (GLM: light: w42 = 3.46, P = 0.484, medium: w24 = 6.19, P = 0.185, strong: w24 = 5.26, P = 0.262; Fig. 2). The same pattern held true for fault bars in the coverts, although slight differences emerged (GLM: light: w32 = 4.36, P = 0.225, medium: w23 = 7.59, P = 0.055, strong: w23 = 6.64, P = 0.084; Fig. 2). The risk of feather breakage was negatively correlated with fault bar prevalence (Spearman’s r =—0.673, n = 27, P = 0.0001) and abundance (Spearman’s r =—0.666, n = 27, P = 0.0002) (Fig. 3). Harmless fault bars, that is those producing feather damage in o2% of the cases (left of dashed line in Fig. 3) occurred at variable prevalence and abundance in different feathers, from being almost completely absent to a high prevalence and abundance. However, fault bars constituting a higher risk of feather damage (those on the right of the dashed line in Fig. 3) occurred at a very low prevalence and abundance on feathers. The proportion of feathers with at least some damage was similarly low across flight feathers (between 3 and 6%; w24 = 5.17, P = 0.270; Fig. 4); however, it was more variable within wing coverts (between 2 and 14%; w32 = 29.82, Po0.0001), being especially high in tertial coverts (Fig. 4). Number of fault bars The number of strong fault bars was equally low in all flight feathers (GLMM: F4,1135 = 1.60, P = 0.172); however, the number of medium fault bars was higher in tertials and scapulars (GLMM: F4,1135 = 2.42, P = 0.0467). Light, and overall fault bar abundance, was higher in scapulars and tertials and slightly higher in external than in internal primaries (GLMM, light: F4,1135 = 12.07, Po0.0001; all: F4,1135 = 8.83, Po0.0001). The same pattern was repeated in coverts, with the differences in fault bar abundance being even more pronounced (GLMM: light: F3,1059 = 48.01, Po0.0001; medium: F3,1059 = 13.23, Po0.0001; strong: F3,1059 = 29.41, Po0.0001; all: F3,1059 = 58.24, Po0.0001; Fig. 1b). Figure 2 Percentage of fault bars producing feather damage. The sample sizes (number of fault bars) for feather groups from left to right were: light: 242, 161, 1031, 272, 287, 409, 544, 400 and 318; medium: 66, 40, 174, 52, 52, 92, 90, 59 and 39; strong: 43, 20, 79, 18, 18, 24, 41, 68 and 37. Figure 3 Relationship between percentage of fault bars producing breakage (risk of feather breakage by fault bars) and fault bar prevalence (left axis) and abundance (right axis). Figure 4 Abundance and prevalence of feather breakage by fault bars along wing flight feathers and coverts. Note the same scale as in Figs 1 and 2 for comparison. The abundance of feather damage was equally low, between 0.03 and 0.06 breaks per feather, among flight feathers (Kruskal–Wallis w42 = 5.09, P = 0.278; Fig. 4), but different within covert feather groups (between 0.02 and 0.17 damages per feather; Kruskal–Wallis w32 = 29.70, Po0.0001), and being especially high in tertial coverts (Fig. 4). Discussion Fault bars were equally or more prevalent on inner wing feathers than on outer ones, consistent with the studies on a stork, passerine and raptor (Jovani & Blas, 2004; Serrano & Jovani, 2005; Sarasola & Jovani, 2006). The fault bar allocation hypothesis proposes that such a pattern is the result of the higher cost of fault bars in these feathers (Jovani & Blas, 2004). Accordingly, we found that the occurrence of fault bars was dependent on their risk of producing feather damage. Fault bars occurred infrequently at intensities or positions prone to undergoing or producing rachis breakage or loss of vane portions. As a result, the prevalence and abundance of feather damage due to fault bars was low and similar along the wing feathers. However, despite the negative association between damage risk and fault bar occurrence across feathers (Fig. 3), the prevalence and abundance of feather damage was not uniform among the feather types studied that is feather damage was highest in the inner coverts, particularly TC and ScC (Fig. 4). Thus, it seems that natural selection was weaker on these inner feathers, which presumably have lower strength demands during fight, and perhaps lower fitness-related costs if they break. We assessed for the first time the probability of feather damage due to fault bars across time, and found no effects of feather age. This result is particularly interesting because we studied feathers ranging from those that were growing to more than 3 years old. Also, the probability of feather breakage was similar between long wing feathers and their corresponding coverts despite the fact that wing coverts are less likely to bend during flight and are exposed less to friction with abrasive materials. Therefore, if a fault bar is going to cause damage to the feather, it seems that this occurs shortly after feather production. This suggests that fault bars have a potential impact on flight performance during the entire period of feather use. Moreover, this finding represents a considerable methodological advantage for future studies, because feathers of different ages can be compared without bias regarding feather damage. This also allows for more valid comparisons between studies conducted with moulted feathers collected in the field and those conducted with whole birds. What do these results explain about the evolution of feathers? Long-distance migration is a challenge for the evolution of bird morphology and physiology, and sandhill cranes cover several thousand kilometres annually between breeding and wintering grounds (Tacha et al., 1992). It thus seems plausible that natural selection has favoured some mechanism to reduce the harmful consequences of increased wing loading and decreased manoeuvrability due to partial or complete feather gaps (Hedenstrom & Sunada, 1999) and their associated wing asymmetry consequences (Thomas, 1993). Thus, it is difficult to imagine a single advantage to the presence of fault bars; the optimal solution is to have no fault bars. However, feather evolution has not eliminated the occurrence of fault bars in birds; as we have found here, fault bars are abundant in cranes and continue producing feather damage. This suggests that the mechanisms by which such feather growth errors are produced, which remain largely unknown, are difficult to avoid. Although natural selection has not eliminated fault bar formation, it has succeeded in reducing to a large extend their occurrence on feathers with a high risk of breaking due to fault bars, in turn reducing damage on feathers that must be in good condition for flight. Fault bars are still produced on important flight feathers, where breakage may result in negative fitness consequences, but in lower numbers. Overall, this shows that natural selection does not ‘select’ optimal solutions (e.g. mechanisms eliminating fault bars), but rather punishes bad designs (e.g. fault bars occurring on feathers that are important for flight). Finally, fault bar occurrence is highly variable among feathers, suggesting that adaptive mechanisms that successfully reduce fault bar occurrence in some feathers are not used in those other feathers with a higher abundance of fault bars. This suggests that either fault bar avoidance mechanisms are costly to implement or that the characteristics of feather growth leading to fault bars have evolved separately in different feather tracts or even at the individual feather level. Acknowledgements This work was supported by a grant from Telefonica Moviles, Vodafone and Amena and a postdoctoral grant from the Secretarıa de Estado de Educacion y Universidades and Fondo Social Europeo to R.J., a postdoctoral I3P contract (CSIC/European Union) to J.B., an NSERC grant to G.R.B. and the Stuart and Mary Houston Professorship in Ornithology to G.R.B. References Baker, K. (1993). Identification guide to European non-passerines. London: Butler & Tanner. Bortolotti, G.R., Dawson, R.D. & Murza, G.L. (2002). Stress during feather development predicts fitness potential. J. Anim. Ecol. 71, 333–342. Hedenstrom, A. & Sunara, S. (1999). On the aerodynamics of molt gaps in birds. J. Exp. Biol. 202, 67–76. Hobson, K.A., Van Wilgenburg, S., Wassenaar, L.I., Hands, H., Johnson, W.P., O’Meilia, M. & Taylor, P. (2006). Using stable hydrogen isotope analysis of feathers to delineate origins of harvested sandhill cranes in the central flyway of North America. Waterbirds 29, 137–147. del Hoyo, J., Elliot, A. & Sargatal, J. Eds (1996). Handbook of the birds of the world, Vol. 3. Barcelona: Lynx Edicions. Jovani, R. & Blas, J. (2004). Adaptive allocation of stressinduced deformities on bird feathers. J. Evol. Biol. 17, 294–301. King, J.R. & Murphy, M.E. (1984). Fault bars in the feathers of white-crowned sparrows: dietary deficiency or stress in captivity and handling? Auk 101, 168–169. Littell, R.C., Milliken, G.A., Stroup, W.W. & Wolfinger, R.S. (1996). SAS system for mixed models. Cary: SAS Institute Inc. Machmer, M.M., Esselink, H., Steeger, C. & Ydenberg, R.C. (1992). The occurrence of fault bars in the plumage of nestling ospreys. Ardea 80, 261–272. Mauck, R.A. & Grubb, T.C. Jr (1995). Petrel parents shunt all experimentally increased reproductive costs to their offspring. Anim. Behav. 49, 999–1008. Møller, A.P., Erritzøe, J. & Nielsen, T. (2009). Frequency of fault bars in feathers of birds and susceptibility to predation. Biol. J. Linn. Soc. 97, 334–345. Newton, I. (1986). The sparrowhawk. Calton: Poyser. Pennycuick, C.J. (1989). Bird flight performance: a practical calculation manual. Oxford: Oxford University Press. Prum, R.O. & Williamson, S. (2001). Theory of the growth and evolution of feather shape. J. Exp. Zool. 291, 30–57. Riddle, O. (1908). The genesis of fault bars in feathers and the cause of alternation of light and dark fundamental bars. Biol. Bull. 14, 328–371. Rohwer, S., Ricklefs, R., Rohwer, V. & Copple, M. (2009). Allometry of the duration of flight feather molt in birds. PLoS Biol. 7, e1000132 Sarasola, J.H. & Jovani, R. (2006). Risk of feather damage explains fault bar occurrence in a migrant hawk, the Swainson’s hawk Buteo swainsoni. J. Avian Biol. 37, 29–35. Serrano, D. & Jovani, R. (2005). Adaptive fault bar distribution in a long-distance migratory aerial forager passerine? Biol. J. Linnean Soc. 85, 455–461. Slagsvold, T. (1982). Sex, size, and natural selection in the hooded crow Corvus corone cornix. Ornis Scand. 13, 165–175. Tacha, T.C., Nesbitt, S.A. & Vohs, P.A. (1992). Sandhill crane (Grus canadensis). In The birds of North America, No. 31. Poole, A., Stettenheim, P. & Gill, F. (Eds). Philadelphia/Washington: The Academy of Natural Sciences/The American Ornithologists’ Union. Thomas, A. (1993). The aerodynamic cost of asymmetry in the wings and tails of birds: asymmetric birds can’t fly around tight corners. Proc. Roy. Soc. Lond. Ser. B 254, 849–854. Velando, A. (2002). Experimental manipulation of maternal effort produces differential effects in sons and daughters: implications for adaptive sex ratios in the Blue-footed Booby. Behav. Ecol. 13, 443–449.