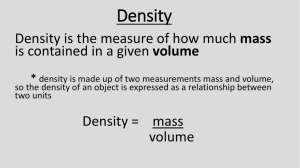Chapter 1 Introduction: Matter and Measurement Chemistry, The Central Science
advertisement

Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; Bruce E. Bursten and Catherine J. Murphy Chapter 1 Introduction: Matter and Measurement Donna Narsavage Heald Siena College Loudonville, NY 2009, Prentice Hall If you have equal masses of the following metals, which will occupy the largest volume? 1. 2. 3. 4. 5. Au, density = 19.3 g/cm3 Pb, density = 11.3 g/cm3 Ag, density = 10.5 g/cm3 Cu, density = 8.92 g/cm3 Al, density = 2.70 g/cm3 If you have equal masses of the following metals, which will occupy the largest volume? 1. 2. 3. 4. 5. Au, density = 19.3 g/cm3 Pb, density = 11.3 g/cm3 Ag, density = 10.5 g/cm3 Cu, density = 8.92 g/cm3 Al, density = 2.70 g/cm3 How many significant figures should be shown for the calculation? 1. 2. 3. 4. 5. 1 2 3 4 5 1.25 0.45 2.734 How many significant figures should be shown for the calculation? 1. 2. 3. 4. 5. 1 2 3 4 5 1.25 0.45 2.734 Estimate the mass of a quarter in grams. 1. 2. 3. 4. 5. 2.5 g 5.5 g 8.5 g 10 g 15 g Estimate the mass of a quarter in grams. 1. 2. 3. 4. 5. 2.5 g 5.5 g 8.5 g 10 g 15 g Estimate room temperature (~72°F) in °C. 1. 2. 3. 4. 5. ~ 15°C ~ 22°C ~ 27°C ~ 32°C ~ 37°C Estimate room temperature (~72°F) in °C. 1. 2. 3. 4. 5. ~ 15°C ~ 22°C ~ 27°C ~ 32°C ~ 37°C Which represents the largest volume? 1. 2. 3. 4. 5. 0.25 L 2.5 x 102 mL 2.5 x 106 L 2.5 x 108 nL 2.5 x 1010 pL Which represents the largest volume? 1. 2. 3. 4. 5. 0.25 L 2.5 x 102 mL 2.5 x 106 L 2.5 x 108 nL 2.5 x 1010 pL