Informed Consent Form Template - Research Study Participation
advertisement

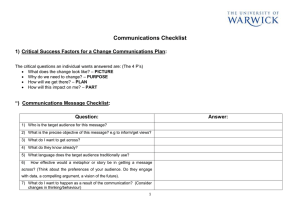
TEMPLATE for an Informed Consent Form (To be customized to each research project. Kindly refer to the checklist for elements required in an informed consent form) Wording in black can be used as is and wording in blue is for guidance. Please delete the latter when submitting the informed consent form. Consent to participate in a research study Include Title of study Investigator: Dr. Address: American University Hospital Cairo Street Beirut, Lebanon Phone: (01) 350 000 ext Site where the study will be conducted: The informed consent should be written in a language understandable by a layperson preferable at the level of 8th grade, should not contain scientific jargon and if it does this should be explained, should be written in the second person singular (addressed to the patient), and should include the following sections: You are being asked to participate in a clinical research study conducted at the American University of Beirut. Please take time to read the following information carefully before you decide whether you want to take part in this study or not. Feel free to ask your doctor if you need more information or clarification about what is stated in this form and the study as a whole. 1) Purpose of the research study and overview of participation Items 1-6, 10-12 and 22-26 of the checklist 2) Any risks as a result of participating in the study Items 7-8 of the checklist 3) Any benefits as a result of participating in the study Item 9 of the checklist 4) Any alternative treatment Item 18 of the checklist Include protocol number (if applicable), Version date and page # If you agree to participate in this research study, the information will be kept confidential. Unless required by law, only the study doctor and designee, the ethics committee and inspectors from governmental agencies will have direct access to your medical records. Item 19-20 of the checklist 6) In case of any adverse event as a result of the study, there will be no compensation to cover such expenses in case it is not covered by a third party or governmental insurance. (This phrase may be modified according to each study / Item 21 of the checklist) Signature section (as listed in items 13-17, 25-31 and 35 of the checklist); Investigator’s Statement: I have reviewed, in detail, the informed consent document for this research study with (name of patient, legal representative, or parent/guardian) the purpose of the study and its risks and benefits. I have answered to all the patient’s questions clearly. I will inform the participant in case of any changes to the research study. _______________________ Name of Investigator or designee Signature Date & Time Patient’s Participation: please add the name of the PI and the contact number in the highlighted section below. I have read and understood all aspects of the research study and all my questions have been answered. I voluntarily agree to be a part of this research study and I know that I can contact Dr. at or any of his/her designee involved in the study in case of any questions. If I feel that my questions have not been answered, I can contact the Institutional Review Board for human rights at . I understand that I am free to withdraw this consent and discontinue participation in this project at any time, even after signing this form, and it will not affect my care or benefits. I know that I will receive a copy of this signed informed consent. __________________________ Name of Patient or Legal Representative or Parent/Guardian Signature Include protocol number (if applicable), Version date and page # Date & Time Witness’s Name (if patient, representative or parent do not read) Witness’s Signature Date & Time Include protocol number (if applicable), Version date and page #