Poster_Fernandez-Tome_Definitivo.pptx
advertisement

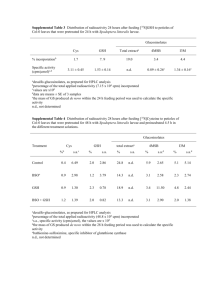
Lunasin, a bioavailable food peptide, exerts protective effects against oxidative stress in human liver cells S. Fernández-Tomé1, S. Ramos2, I. Cordero-Herrera2, I. Recio1, L. Goya2, B. Hernández-Ledesma1* 1 Instituto de Investigación en Ciencias de la Alimentación (CIAL, CSIC-UAM), Madrid, Spain. 2 Instituto de Ciencia y Tecnología de Alimentos y Nutrición (ICTAN, CSIC), Madrid, Spain. * b.hernandez@csic.es BACKGROUND METHODS • Lunasin is a 43-amino acid peptide which chemopreventive properties have been demonstrated both in vitro and in vivo studies1. Effects on cell viability and biomarkers of redox status Lunasin Direct effects • Bioavailability studies have shown that lunasin remains intact in a high percentage during its passage through the gastrointestinal tract reaching different target organs, such as liver, in an intact and active form2. Analysis t-BOOH Chemical-induced oxidative stress • Reactive oxidative species (ROS) accumulate in cells playing a key role in the induction and progression of several diseases related to the oxidative damage to liver tissues3. Lunasin + OBJECTIVES t-BOOH Chemo-protective effects HepG2 cells To evaluate the potential chemo-protective effects of lunasin against tert-butyl hydroperoxide (t-BOOH) induced oxidative stress in human liver HepG2 cells. Stability of lunasin Lunasin: incubation times (h) Cell supernatants To investigate the stability of lunasin, and to identify lunasin derived fragments released in HepG2 cultures. RESULTS Table 1. Direct effects of lunasin on non-stressed HepG2 cells. Cell viability was measured by the crystal violet assay (n = 12). Fluorescence units correspond to intracellular ROS generation (n = 8). GSH intracellular levels were calculated as nmoles of GSH per mg of protein (n = 6). All results are mean ± SD, and are represented as percent of control non-stressed cells. *, significantly different from control cells. Cell viability (% control) Intracellular ROS generation (% control) Intracellular GSH levels (nmol/mg prot, % control) Control 100.00 ± 8.41 100.00 ± 7.02 100.00 ± 7.85 0.5 μM lunasin 107.15 ± 2.73 76.17 ± 5.59 *** 118.83 ± 21.04 1 μM lunasin 111.37 ± 3.52 63.98 ± 5.61 *** 123.74 ± 10.04 * 5 μM lunasin 109.88 ± 3.68 63.61 ± 5.41 *** 122.13 ± 12.31 * 10 μM lunasin 107.26 ± 8.02 71.75 ± 10.82 *** 125.62 ± 6.18 * A *** 50 150 ### ### ### ### 100 50 0 0 0.5 1 5 t- BOOH 0.5 *** 250 # ### 150 100 50 1 5 0.5 1 5 100 ### 50 10 ### ### 5.00E+07 ### *** 4.00E+07 t- BOOH 0.5 E *** 300 250 200 # ## ## 150 1 5 10 3.00E+07 Lunasin (μM) ## 100 50 350 F *** 300 2.00E+07 250 200 ### 150 1.00E+07 ### ### 100 0.00E+00 ### 50 0 0 C t- BOOH 0.5 1 5 10 C t- BOOH 0.5 Lunasin (μM) Lunasin (μM) Figure 2. Protective effects of lunasin on t-BOOH-stressed cells. (A) Cell viability was measured by the crystal violet assay (n = 12). (B) Fluorescence units correspond to intracellular ROS generation (n = 8). (C) GSH intracellular levels were calculated as nmoles of GSH per mg of protein (n = 6). (D) GPx activity was calculated as mUnits per mg of protein (n = 6). (E) CAT activity was calculated as mUnits per mg of protein (n = 4). (F) Protein carbonyl content was calculated as nmol per mg protein (n = 4). (G) Caspase-3 activity was calculated as Units per μg of protein (n = 4). All results are mean ± SD, and are represented as percent of control non-stressed cells (C). *, significantly different from control non-stressed cells. # , significantly different from t-BOOH-stressed cells. 1 5 10 2 Lunasin (μM) 250 (U/μg prot, % control) t- BOOH 6.00E+07 C 0 C C 10 Caspase-3 activity 0 (mU/mg prot, % control) 350 200 7.00E+07 Lunasin (μM) CAT (mU/mg prot, % control) GPx Lunasin (μM) D 150 Peak Area (AU) 0 C 10 (nmol/mg prot, % control) t- BOOH Protein carbonyl content C 300 (nmol/mg prot, % control) 100 *** GSH ### ### B 200 (% control) ### ### ROS generation (% control) Viable cells 250 A D Figure 1. Morphological analysis of HepG2 cells. Representative images of (A) non-stressed cells pre-incubated with medium for 20h, (B) t-BOOH-induced (400 μM, 3h) cells pre-incubated with medium for 20h, and t-BOOHinduced (400 μM, 3h) cells pre-incubated for 20h with (C) 0.5 μM lunasin, and (D) 5 μM lunasin. Treatment of non-stressed cells with lunasin (0.5-10μM) for 20h did not damage cell integrity, decreased intracellular ROS generation, and enhanced cytosolic levels of GSH. 150 C B 6 12 20 Incubation time (h) G *** 200 Lunasin 150 ### 0 0.5 1 F3, f(29-43) F4, f(26-43) F5, f(25-43) ### 50 t- BOOH F2, f(30-43) ### 100 C F1, f(32-43) 5 10 Lunasin (μM) Pre-treatment of t-BOOH-induced cells with lunasin (0.5-10μM) for 20h restored cell viability and GSH levels, quenched intracellular ROS and carbonyl groups generation, regulated antioxidant enzymes activities, and prevented the apoptotic effects induced by disruption of the redox steady-state. Figure 3. Stability of peptide lunasin in medium added to HepG2 cells and identification of lunasin derived fragments. Relative amount (expressed as peak area) was calculated from extracted ion chromatogram of the molecular ion of lunasin m/z 1257.5 (charge +4), f(32-43) (F1) m/z 1324.5 (charge +1), f(30-43) (F2) m/z 1565.5 (charge +1), f(29-43) (F3) m/z 1693.8 (charge +1), f(26-43) (F4) m/z 1034.1 (charge +2), and f(25-43) (F5) m/z 1102.7 (charge +2) in medium incubated with 10 μM lunasin and collected after 0, 2, 6, 12, and 20 h-incubation. Content of lunasin in the medium of HepG2 cells notably decreased with the incubation time, while five lunasin derived fragments were generated. CONCLUSIONS Lunasin prevented the increased ROS generation and the depletion of GSH, modulated the GPx and CAT activities, and evoked a decline in carbonyl groups and a recovery from cell death by apoptosis. Five major lunasin-derived fragments released by cell metabolism have been identified as part of cellular response to lunasin treatment. Lunasin and its derived-fragments, at physiological concentrations, might confer a significant chemoprotection against oxidative stressassociated liver disorders. REFERENCES (1) Hernández-Ledesma, B., Hsieh, C.-C., & de Lumen, B. O. (2013) Chemopreventive properties of peptide lunasin: a review. Protein Peptide Lett. 20, 424-432. (2) Hsieh, C.-C., Hernández-Ledesma, B., Jeong, H.J., Park, J.H, & de Lumen, B.O. (2010) Complementary roles in cancer prevention: protease inhibitor makes the cancer peptide lunasin bioavailable. PLoS ONE 5, e8890. (3) Vitaglione, P., Morisco, F., Caporaso, N., & Fogliano, V. (2004) Dietary antioxidant compounds and liver health. Crit. Rev. Food Sci. Nutr. 44, 575-86. ACKNOWLEDGEMENTS This work was supported by projects AGL2010-17579, AGL2011-24643, CSD2007-00063 from Programa Consolider-Ingenio from the Spanish Ministry of Education and Science (CICYT), FP7-SME-2012-315349 (FOFIND), and FEDER-INNTERCONECTA-GALICIA (ENVELLEFUN). The authors are participants in the FA1005 COST Action INFOGEST on food digestion. I. C. -H. and S. F. -T. acknowledge Ministry of Economy and Competitiveness (MINECO) for their FPI fellowships, and B. H. -L. acknowledges MINECO for her “Ramon y Cajal” contract.