BLM 8-1 Outer Electron Worksheet-Sasso 2004.doc
advertisement

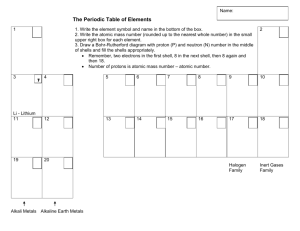
DATE: NAME: CLASS: BLM 8 - 1 SCIENCE INQUIRY I The Outer Electrons Goal • Demonstrate your knowledge of shell diagrams for the first 20 elements. What to Do Demonstrate your knowledge by answering the following questions in the spaces provided. Questions 1. In the spaces provided, draw shell diagrams for the five named elements. 2. Complete the shell diagrams for the elements in Group 1 (alkali metals) indicated below. 3. What similarities exist among the shell diagrams for the alkali metals? 4. Examine the rows of the periodic table. As the rows increase, what happens to the number of shells of electrons? 5. How many electrons are there in the outer shell of a rubidium (Rb) atom?_ 6. How many electron shells would you expect to find in a rubidium atom? _ 7. Identify the elements whose shell diagrams are shown below. Place the name for the element in the spaces provided. B D Copyright © McGraw-Hill Ryerson Limited. Permission to reproduce this page is granted to the purchaser for use in her/his classroom only. 463