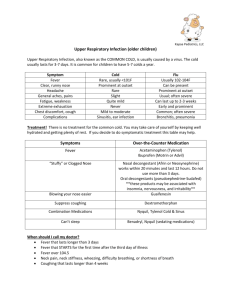
Document 15364879
advertisement

Arthropod borne Diseases By Dr. Sabry Ahmed Salem Prof. of community medicine Environ mental health & occupational medicine Classification According to causative agent I. Bacterial : - Tick borne relapsing fever - Louse borne relapsing fever II. Viral: - Yellow fever - Rift valley fever - Dengue(break bone) fever - Arbovirus encephalitis - Sandfly fever III .Parasitic: - Malaria - Filarial - leishmaniasis IV. Rickettsial: - Typhus group (epidemic, endemic and scrub typhus). - Spotted fever (rocky mountain fever). - Trench fever. Plague Definition: Acute Infectious – insect – borne disease gram (-)ve bacilli. Causative: Yersinia (Pasteurella pestis) , Gram –ve, bipolar stained and killed by heat, sunlight, and disinfectants. Reservoir of infection: - Rats and other rodents (wild) flea. - Domestic rodents through fleas outbreak (epizootic) - Death of rats, fleas leave rats and causes epidemic of plague. Vector: rat flea (xenopsylla cheopis). -Rat to rat then them rat to man Mode of transmission: 1. Flea faeces of flea: lead to contamination of skin abrasions. 2. Flea bite. 3. Droplet infection: from man to man in pneumonic cases. Incubation period: International period is 6 days. Clinical picture: 3 types: 1. Bubonic plague: lymph nodes usually inguinal 2. Septicemic plague. 3. Pneumonic plague: fatal. Diagnosis: 1. Clinical picture. 2. Laboratory: sample from affected blood, lymph node or sputum (bipolar-stain) bacilli (G-ve) with characteristic bipolar staining. Prevention: 1- General prevention Rodent control: by – removal of breeding places. - Rat-proof buildings. - Trapping. - Insecticides. - Fumigation by SO2 & HCN of cargoships . Flea control: by Ventilation and dust control. Removal of breeding places. Insecticides (D.D.T dusting): dusting breeding places and rats holes and runs 1- Specific protection: by 1- Active immunization : to risk- groups a- Otten's vaccine. Live- attenuated , single dose , 1 ml S.C, causes about 6 months- protection. b- Haffkin’s vaccine Killed vaccine, 2 doses: 0.5 ml (after 7-10 days) 1 ml Sc gives few months protection. 2- Chemoprophylaxis: to contacts and at-risk group. Tetracycline 250mg/6hrs. for 6 days. 3- International (Quarantina measures): Protection of ports against rodents. Deratting certificate from cargoship (fumigated by SO2, HCN) and valid for 6 months. Control: Case: Notification, isolation, heavy dusting with DDT 10%, to any place where fleas may be found. Treatment by chemotherapy and release when clinically free. Contacts: - Dusting with DDT 10%. - Surveillance for 10 days. -Chemoprophylaxis: tetracycline 250 mg/ 6 hrs for 6 days. Epidemic measures: -Mass Flea control before rat control. -Chemoprophylaxis of at-risk groups. -Case finding management. * Causative agent: plasmodium (protozoon) having four species 4 species 1- P. Vivax - Benign tertian (vivax) malaria 2- P. Ovale - Ovale malaria (Benign tertian, less common). 3- P. Malariae 4- P. Falciparum * Vector: Extrinsic I.P: - Quartan malaria. - Malignant malaria. - Female Anopheles mosquito with sporozoites in their salivary glands. Average: 2 weeks. * Reservoir of infection: a. Infected person (patient) having gametocytes in his peripheral blood causes infection if infective to mosquito vector ♀. b. Patient can be a carrier of several plasmodial species at the same time. * Period of infectivity: For many years or life so long as gametes are present in the blood and no treatment is given. Relapses: In P. vivax and ovale may occur more than 3 years after the first attack. In P. falciparum disappears within 1-2 years. P. malariae shows prolonged low. Level asymptomatic parasitism. * Mode of transmission: a- Insect- bite by Infective female Anopheles mosquito sporozoites ( the infective stage in the salivary glands of mosquito). b- Direct- transmission: by inoculation of contaminated blood (transfusion). * Incubation period: a- average: 2 weeks - falciparum malaria (12 days) - vivax malaria ( 14 days) - quartan malaria (28 days) - ovale malaria (17 days) •Clinical picture and diagnosis 1- Attacks of rigors, fever then sweating: - Rigors or cold shivers with fever ( ½ -1 hour). - Hot stage; fever (1 to 4 hrs.) - Sweating stage: Profuse sweating (1-4 hrs) the above attacks are due to destruction of R.B.Cs and release of pigments and toxins. 2- Anaemia (hemolytic) 3- Hepato- splenomegaly: (due to engulfed parasites by the recticulo-endothelial cells hypertrophy). 4- Malignant malaria (P. faciparum) may be: a-Attacks are not well defined and fever may be continuous or irregular. i.e. atypical attack b-Clumping of the affected R.B.Cs leads to occlusion of blood capillaries causing lesions according to the affected area. C. May be severe and fatal taking different forms i- Cerebral form: headache, fever, convulsions and coma. ii- Algid form: collapse and peripheral circulatory failure. iii- G.I.T form: severe diarrhea or dysentery dehydration. d. Black water fever. Acute intravascular haemolysisi of R.B.Cs caused by repeated attack or prolonged course of P. falciparum infection and manifested by: hemolytic anemia (+++), hemolytic jaundice, high fever, hemoglobinuria (Dark Red urine) and high Mortality Rate. 5- Nephrosis, specially in children may occur with P. falcipartum infection (pus And casts in urine). 6- Abortion or congenital infection when the pregnant is infected with malaria. * Diagnosis: Clinically: Acute attacks of chills, fever and sweating are suggestive. Laboratory: blood film examination for the parasite during the febrile attack. * Prevention and control of malaria: I- Control of human reservoir (cases) 1- Case- finding: During health appraisal by blood films and clinical examination. During survey studies and malaria campign for chronic cases and asymptomatic infections. 2- Treatment of diagnosed cases by proper chemotherapy. 3- Re-examination: after treatment. II- control of vector: 1. Eradication of breading places (water collections). 2. Control of breading places (larvae) collections) 3. Destroying the adult mosquito by insecticides with temporary (inside the building) or permanent (residual) outside the buildings. III- Protection of man: 1. Prevention of mosquito to reach man by: screening, Animal barrier & netting. 2. Protection of man against mosquito bites by, protective clothes, netting of beds & application of repellents. 3. Specific prevention by chemoprophylaxis. * Chemoprophylaxis: for visitors to endemic areas: Chloroquine : Antimalarial drug is given in suitable dose one week before traveling and continue for 2 weeks after leaving Chloroquine is the drug of choice and the prophylactic dose may be one tablet / week. * Eradication of malaria: Is the elimination of malaria in a certain locality based on destruction of the mosquito vector in a timelimited period. Methods of implementation: i.e. requirements: Survey study of vector to put a plan of work. Mass application of insecticides (residual) for all inhabited premises of the locality to be repeated yearly for 3 years or more. 1. Supporting eradication by mass, case finding and treatment and chemoprophylaxis and health education of the public. 2. Follow- up of work by survey study to check fulfillment (evaluation) and maintenance of eradication. (i.e. to check progress of eradication) • Malaria survey: It a field study to assess the magnitude of malaria problem in a given locality and study the ecological factors determining spread and endemicity of the disease. I. Objectives: - Measuring the magnitude of the problem . - Studying the ecological factors. - Planning for prevention & control. II. Steps: - Planning. - Preparation. - Investigations. - Tabulation of data. - Analysis of data. - Report- writing. III. Investigations: Locality. - Infection in man. Insect vector. *Investigations 1- Locality: - Map showing water channels and collections, cultivated land and houses. - Climate. - Population data. 2- Infection in man: Examination of children aged 2-9 years for finding malariometric indicies. Or malaria indices a- Spleen index: % of examined children with splenomegaly. N.B: Non specific and ONLY valid in areas free of parasites associated with splenomegaly. b- Parasite index: % of examined children having malaria parasites in blood. * This is the Direct specific index of malaria infection. 3- Insect vector (biological environment) a- Study of breeding places. Sampling of water collections in the area to find anopholes larvae and pupae. b- Study of adult mosquitoes Density and habits of anopheles mosquitoes in the building of the area. Collection of mosquitoes to be examined for specific malaria indices which include: Oocyst index: % of examined anopheles mosquitoes having Oocysts in stomach wall. Sporozoite index: % of examined female anopheles mosquitoe, having sporozoites in salivary glands. (more valid and specific for the infection rate of the mosquito I. Oocyst index: % of examined anopheles mosquitoes having Oocysts in stomach wall. II. Sporozoite index: % of examined female anopheles mosquitoe, having sporozoites in salivary glands. (more valid and specific for the infection rate of the mosquito vector). • Malariometric indices: - 2 for man - 2 for mosquito a- in children aged 2-9 years - Spleen index - Parasitic index. b- For mosquitoes - Oocyst index. - Sporozoite index. Filariasis Definition: Parasitic, disease of lymphotics and lymph nodes, may ending by elephantiasis. Causative agent: Wuchereria Bancrofti (is the most important). Reservoir: Man: as a case: Gravid female worm puts microfilaria which appear in peripheral blood at night. Vector: Mosquito Culex pipiens (Insect bite): the mosquito takes microfilariae with blood meals). Mode of transmission: by mosquito (culex) bite Clinical picture & pathology: Acute stage: - Lymphadenitis and local lymphagnitis Chronic stage: a. Lymphatic dilatation: i.e. lymph varies. b. The Lymphatic Vs. rupture chyeuria. Chylothorax, chylous ascites & chylocale. c. Then Elephantiasis: commonly legs and scrotum. Diagnosis: Clinical picture: suggestive in endemic areas. Laboratory: Blood film (at night) shows microfilariae. Prevalence: Endemic in Egypt at Giza and Rasheed as foci of endemicity due to presence of reservoir of infection (cases) and breeding of mosquito vector. Prevention: 1- Mosquito control: (Place, larvae, adult mosquito) a. Eradication of breeding places. b. Control of breeding places by fish (Gambusia affinis ) and insecticides (larvae). c. Adult mosquito control: by insecticides for temporary or residual affect, outside and inside houses. 2- Man protection against mosquito bite by : a. Screening. b-Animal barrier. C-Protective clothes and netting of beds. a. Repellents application. Control of filariasis -Case finding. - Treatment (Hetrazan, filiran). Filariasis compaign: In endemic areas: Survey study. Mass case finding and treatment. Vector eradication or control. Arthropod borne Viral diseases Caused by RNA virus of (Arboviruses) & Arthropod – borne infection Reservoir of infection: A- Animals - Rift valley fever - Hemorrhagic fever. B- Man - Encephalitides - Jungle yellow fever. - Urban / rural yellow fever - Dengue. - Sandfly fever Vectors: Biting insects as mosquitoes, sandfly and ticks. Yellow fever Definition: Acute infectious arthropod- borne viral disease. Causative agent: Yellow fever virus (arbovirus). Reservoir of infection: Man (cases): in urban / rural yellow fever. Monkey: in jungle yellow fever. Vector: female Aedes aegypti (♀) in Urban type. Female aedes African usually in Africa, Jungle yellow fever. Hemagogus in America. Mode of transmission: Bite of infective mosquito How man acquires infection 1. Entering the jungle: Y. f is endemic. 2. Monkey strays into human settlement and bitten by A-aegypti mosquito causes man infection. 3. Flying mosquitoes outside the forest for short distance causes man infection. Incubation period; About 3 –6 days. (6 days international) Susceptability: All ages and sexes get urban Y.F. Young adult males who work in the Jungle if get infection it is occupational disease. Clinical picture: Hepatitis with liver necrosis, kidney dysfunction. Fever, Jaundice, hemorrhage and albuminuria. Diagnosis: Clinical picture. Laboratory : by demonstration of antibodies (neutralizing, complement fixing and haemoaglutination Abs.). Immunity: Natural acquired immunity after disease, absolute immunity. Artificially induced after active immunization by 17-D vaccine produces absolute immunity for at least 10 years. Prevention: 1- Mosquito control: In jungle Y.F. is impractical. In urban Y.F : anti- larval and anti- adult measures. 2- Specific prevention by vaccination a- 17-D. vaccine: live- attenuated, prepared in chick- embryo, 0.5 ml SC, protection after 10 days for at least 10 years. b- Dakar vaccine: Live otten. / prepared in mouse-brain, by skin scarification may lead to encephalitis. Not used on international level by WHO. 3- International measures To prevent introduction of Y.F from endemic areas to recipient areas (e.g Egypt). 1- valid international vaccination certificate is required for - Travelers between endemic and recipient areas. - Aircrafts, using airports of endemic areas and employees. Validity : begins after 10 days of vaccination. And lasts for 10 years. If the certificate is not valid (i.e. not available or the travelers arrive before 10 days of vaccination) isolation until the certificate becomes valid with maximum 6 days. b- Disinfections of any air craft leaving an endemic area for receptive area by aerosol spray of insecticide: shortly before and also on arrival if necessary. c- Quarantine of imported monkeys at receptive areas. Rift valley fever (R.V.F) -Acute viral, zoonotic arthropod- borne disease was introduced into Egypt in 1977 from East & south Africa causing an outbreak in animals and was transmitted to man. -Causative agent: specific virus (Phlebovirus subgroup). -Reservoir: cattle & sheep. -Vector: Culex mosquito. - Mode of transmission: 1.Culex bite. 2.Handling of diseased animals or their tissues (occupational infec) -I.P: 3-7 days. Clinical picture (C/ P): Mild form: fever, influenza. Like picture and usually selflimited. Severe form (rare): haemorrhage, liver necrosis, retinal damage and encephalitis. Diagnosis: 1- Clinically : nonspecific. Laboratory: + ve serum antibodies. Prevention: 1. Vector control or eradication. 2. Protection of man against mosquito bite. 3. Protection of man against occupational infections. Dengue (Break bone F.) -Acute infectious insect- borne disease -Causative agent: Dengue virus. -Reservoir: Man (case). -Vector: Aedes aegypti. -Mode of transmission: insect bite. -Clinical picture: -Severe pain in the joints, bone and muscles for some days followed by recovery. -Dengue F. may be associated with hemorrhagic manifestations and renal involvement (hemorrhagic dengue). •Diagnosis: Clinically: nonspecific. Laboratory: +ve serum antibodies. * Prevention: Vector control or eradication. Protection of man against bite of mosquito. Leishmaniasis A group of diseases caused by a protozoon leishmania is classified into: 1. Cutaneous leishmaniasis. 2. Viscereal leishmaniasis. 3. Muco-cutaneous leshmaniasis. I- causative agent: Cutaneous L caused by L. Tropica. visceral L by L. Donovani. muco-cutaneous by L . braziliensis. II-Reservoir of infection: - C.L : man -V.L:man and do -M.L.: man and rodent III- Vector: Phlebotomus papatassi(sand fly) V- Mode of transmission: -Cutaneous L. through Sand fly bite & contact with skin lesions of the case. -Visceral L.due to infective sandfly bite. -Muco-cutaneous L. due to infective sandfly bite. IV- Clinical picture: 1. Cutaneous L. shows Single or multiple ulcerating lesions on the exposed skin. 2. Kala-azar In adults. Prolonged fever, splenomegaly, hepatomegaly, lymphadenopathy, cachexia & death of untreated cases. In infants: affects young children and gives the same picture as adults. 1. M.C Leishmaniasis: Ulcerating skin lesions (nose & mouth) destruction. VI- Diagnosis: a. Clinical picture: suggestive in endemic areas. b.Laboratory: - Stained smear (from organs, ulcer and blood) shows L. bodies. - Culture if indicated. Prevalence of leishmaniasis : * Alexandria region has an endemic focus. * Clinical infection leads to lasting immunity. * L. donovani infection causes cross immunity. Prevention of Leishmaniasis (L. Tropica, L. Braziliensis) 1. Sandfly control. 2. Protection of man against sandfly bites. 3. Control or destroying dogs and rodents. 4. Health education of the public to avoid contact with skin lesions of others. 5. Specific prevention by trial of live- L. tropica vaccine in endemic areas. Control of Leishmaniasis Early case finding (Case-finding) and treatment Covering skin lesion to avoid infection of the vector and contacts. Disinfection of the contaminated objects of the case. Health education of the case and contacts. N.B: Prevention of L. donovane includes: Control of sandfly. Protection of man against phlebotomus paptassi bite. Rickettsial diseases Are classified into 3 groups: I- Typhus group Ag organism reservoir - Epidemic typhus R.prowazeki - Endemic typhus R. mouseri - Scrub typhus R. Tsutsugamushi Man Rat vector, proteus louse, Ox 19 Flea, Ox19 Rodents Mite, Ox K II- Spotted fever group “Scrubtyphus” - Rocky mountain S.F Ox19, Ox2 R.ricketsi Dogs & Rodents - Boutonneuse fever Ox2 R. conori Rodents Tick Ox19, - Rickettsial pox R. Akari Mice Mite III- Trench fever R. Quintana Man Lous - Fever coxiella burnetti Rats ticks. Tick Transovarian transmitted R-diseases Organism transmitted to progeny through the ova. e.g. 1- Hard tick - American spotted fever. - Boutonneuse fever - Q- fever 2- Soft tick Q- fever. 3- Mite (trambicula akamushi) scrub typhus.