Blackbody Radiation and the Ultraviolet Catastrophe The Photoelectric Effect
advertisement

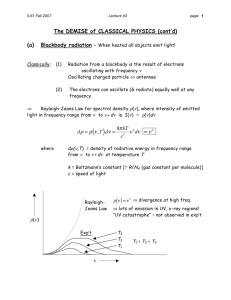
Blackbody Radiation and the Ultraviolet Catastrophe The Photoelectric Effect Dr. Mahmoud Ahmad 2.2 Blackbody Radiation Let’s take another look at the electromagnetic spectrum. Blackbody radiation* is electromagnetic radiation emitted by objects in thermal equilibrium at a non-zero (absolute) temperature. If we claim to understand electromagnetic radiation, we should be able to explain blackbody radiation. We will discuss blackbody radiation in more depth in chapter 9, when we have the mathematical tools to handle it. We’ll skim the mathematics presented in this chapter. *The title of this section, but not really what it is about. All objects emit radiation. For many real-world objects, the radiation can be approximated by blackbody radiation. The characteristics of the radiation depend on the properties of the object and conditions such as temperature. Not surprisingly, the ability of an object to absorb radiation is closely tied to its ability to absorb radiation. Physicists like to study idealized scenarios. It would be nice to find a system where the emitted radiation was independent of the details of the radiating object. A blackbody is an example of an idealized scenario. It is both a perfect absorber and perfect emitter of radiation. As its name suggests, it might be black. “But wait! If the object is black, how can it be a good emitter of radiation?” Good question. Who knows the answer? Just because an object is black does not mean it emits no radiation. Another objection—what do you mean “perfect?” How can you make anything perfect? You can make an extremely good approximation to a blackbody by simply making a small hole in an opaque box. http://www.egglescliffe.org.uk/physics/astronomy/blackbody /bbody.html Who should understand blackbody radiation? Physicists. Astronomers. Meterologists. Politicians. “The principles of blackbody radiation apply quite well to the Sun, stars, and planets. Each can be treated as a radiating blackbody, giving off energy at a rate commensurate with its temperature. Therefore, we can apply this model to finding the rate of energy output (power) from the sun, and other stars. Once we know the power coming out of a star at a given temperature we can use some simple geometry and algebra to determine the radii of distant stars.” http://www.cosmicshell.com/~miranda/lab/BBradiation/BBradiation.html dead link January 2005? “The principles of blackbody radiation are at the heart of both the most simple and the most complex climate models, including the general circulation models (GCMs) which are run on supercomputers and used for predicting global climate change. The principles of blackbody radiation also form the foundation for the science of remote sensing. Passive and active sensing of the earth's surface and atmosphere usually take advantage of the radiative spectrum, measuring intensity of emitted shortwave, visible, longwave, and radiowave radiation. To understand global warming or the workings of meteorological satellites which monitor climate change, we need to understand the principles of blackbody radiation.” http://www.imsa.edu/edu/geophysics/atmosphere/energy/energy1.html E&M radiation enters cavity. Radiation is absorbed by cavity walls. The energy is then emitted by the walls. Only radiation which “fits in” the box can “live” inside, and eventually exit the cavity. The Ultraviolet Catastrophe Using the conceptual design on the previous slide, one can make a blackbody and investigate its radiation. The intensity also depends on the temperature of the blackbody. Why would you guess the blue curve corresponds to the hotter blackbody? A range of colors is emitted. Intensity depends on frequency. Solar Spectrum Intensity (W/m2/m m) 2.50E+03 ASTM E490 Air Mass Zero solar spectral irradiance is based on data from satellites (1999) 2.00E+03 1.50E+03 1.00E+03 5.00E+02 0.00E+00 0 1 2 3 4 WaveLength (micro-meters) 5 We think we have a basic experimental understanding of the measured properties of blackbody radiation. How about coming up with a theoretical* understanding? Rayleigh and Jeans in the late 19th century developed a mathematical description of blackbody radiation by modeling it with standing waves set up inside a cavity. Beiser gives the result and a brief “derivation.” Don’t worry about the mathematics for now. We will return to the math in That’s a “pi” in the Chapter 9. Here’s the result: 8πkT 2 U(f) df = f df . 3 c font I’m using! *“The essential fact is that all the pictures which science now draws of nature, and which alone seem capable of according with observational facts, are mathematical pictures.”—James Jeans 8πkT 2 U(f) df = f df . 3 c U(f) is the energy per unit volume inside the blackbody. T is the temperature of the blackbody, k is Boltzmann’s constant, and f is the frequency of the radiation emitted. We should be able to compare this theoretical formula with the experimental data shown previously. Beiser uses the Greek “nu” symbol for frequency. It is too easy to confuse with v for velocity. Let’s always use f for frequency in this class. theory experiment well… The Ultraviolet Catastrophe! What’s wrong with the theory? Rayleigh and Jeans assumed the radiation was absorbed and emitted by oscillators in the blackbody walls. A valid assumption. This is not as odd an idea as it may sound at first. Light is E&M radiation. E&M radiation can accelerate electrons. Electrons in the atoms of the blackbody wall will act like little balls on springs (harmonic oscillators) when you “pull” on them with light. Electrons absorb light energy. Now they are “excited.” After a very brief time, they will get rid of their excess energy. The excess energy may come out at any valid oscillator frequency, i.e., at any frequency of light corresponding to a valid oscillator energy. Rayleigh and Jeans assumed that the oscillators could later A valid assumption.* emit their energy at any frequency. Rayleigh and Jeans assumed that only wavelengths which could “fit inside” the cavity could exist there. A valid assumption. Rayleigh and Jeans assumed that the radiation exiting the cavity was the same as the radiation inside. A valid assumption. *according to classical physics Planck spent many years investigating blackbody radiation, and discovered that he could explain the blackbody radiation distribution by assuming the blackbody to be made up of an enormous number of oscillators, with each oscillator vibrating at a fixed frequency, but with a wide range (from 0 to infinity) of possible frequencies.* However, the oscillators could not take on any arbitrary frequency. Instead, they could oscillate only in integral multiples of a frequency f which depended on the blackbody temperature.** *Same as Rayleigh and Jeans, so far. **A revolutionary idea. These oscillators emit energy in units of hf, which Planck called "quanta" of energy. A quantum of energy is E = hf, and h is called Planck's constant This h is the same constant which will appear in the next section in the equation for the photoelectric effect. The fact that the oscillators in the cavity walls can interchange energy with standing waves only in units of hf is a dramatic departure from classical physics. Planck’s theory explained blackbody radiation, but even Planck believed that later on somebody would reconcile blackbody radiation with classical physics.* “...the whole procedure was an act of despair because a theoretical interpretation had to be found at any price, no matter how high that might be.”—Max Planck *Nevertheless, Planck won the 1918 Nobel prize for his discovery of quanta. Here is Planck’s formula for blackbody radiation: 8πh f3 U(f) df = 3 hf df . c e kT 1 We’ll derive it in Chapter 9. Planck was right to be suspicious of it, because when he found it, there was no theoretical basis for it. However, it does describe blackbody radiation accurately. Show that at low frequencies Plank formula becomes as Rayleight Jeans formula? Answer Page61 Oscillators can oscillate only in integral multiples of some fundamental frequency. (Chapter 5) These oscillators emit energy in units of hf, called "quanta" of energy. A quantum of energy is E = hf. (Chapter 2) For your interest (not for test): 500K or less -- essentially all of the radiation is at frequencies less than those of visible light. (Infrared – “ROY G. BiV”) The blackbody is black. 2000K -- visible light of appreciable intensity, but mostly at the red (low frequency) end of the spectrum. 3000K -- red predominates, but significantly more blue. This is about the temperature of an incandescent lamp filament. 6500K -- distribution is more nearly uniform, therefore "white hot." Example -- the surface of the sun. 10000K and above -- blue light predominates. "Blue hot." Example -- some of the hotter stars. 2.3 The Photoelectric Effect Heinrich Hertz in 1887-8 studied the photoelectric effect and generated E&M waves to verify Maxwell's theory of the electromagnetic nature of light. Hertz observed that a spark would jump more readily between two metal spheres when their surfaces were illuminated by the light from another spark. Any ideas why that should happen? The actual experimental setup was more complex than sparks and a couple of metal spheres, and allowed for a number of different experiments which verified the electromagnetic nature of light. An example of the kind of apparatus used to study the photoelectric effect (but not Hertz’s apparatus) is described in your text. Start with a glass tube. Insert a couple of metal plates, with vacuum feedthroughs carrying wires to the outside. Pump out “all”* the air and other gases. *You never get all the gases out, but you can get most out. - + x V A Seal off the tube (or keep pumping to maintain a vacuum). Connect the metal plates to a variable high voltage power supply. + is the anode and - is the cathode.* Connect an ammeter to measure the current. *Hint for remembering which is which. Cathode ray tubes have electron guns. Electrons come from the cathode. Electrons are negative. Therefore the cathode is negative. The “other one” must be positive. Chemists must have been confused when they named cations and anions. - + x V A Light striking the anode causes electrons to be emitted from the anode. The electrons are emitted with kinetic energy, and some of them reach the cathode (in spite of its negative voltage). You can measure the current with the ammeter. “Wait a minute—aren’t electrons attracted to + and repelled from -?” They are, but if their initial KE is large enough, they can reach the cathode anyway. - + x V A You can increase the retarding potential (make the cathode voltage more negative relative to the anode) and see how that affects the current. - + x V A You can make the cathode voltage negative enough so that no photoelectrons reach the cathode. (When the cathode reaches the "extinction voltage," no electrons are detected there.) So, what's the big deal. Light carries energy. Energy is transferred to electrons in the anode. They escape the metal anode. If they get enough energy, they can even reach the cathode. Classical physics explains this “perfectly.” Hertz’s experiments with the photoelectric effect confirmed that light consists of electromagnetic waves… …until you looked at the details. Remember, ρ 2 2 Power ω A vp. 2 What does this predict? Predictions, based on classical theory: (1) For a fixed light frequency, the PtransmittedA2; i.e., the power should be directly proportional to the intensity. The energy of the electrons coming out therefore ought to be directly proportional to the intensity (brightness) of the light. (2) Similarly, for a fixed light intensity, KEelectron f2. (3) The extinction voltage ought to depend on the f2, or on the intensity of the light. (4) As Beiser shows, it should take a "long" time for the electrons to accumulate enough energy to escape. (5) I can't see anything here that says there ought to be either a minimum light intensity which causes emission of photoelectrons or a maximum photoelectron energy. Let’s put these in a table, with shorthand notation: Predict PA2, PI Eelectron Ilight Eelectron flight2 Vextinction flight2 tescape = “very long” no limit to electron KEmax, no Imin to produce e- Observe Here's what we actually observe: (1) The number of electrons emitted, but not their energy, depends on the brightness of the light. (2) The electron energy is proportional to the first power of the frequency of the light (not the square). (3) The extinction voltage depends on the first power of the frequency of the light. (4) Electrons are emitted almost right away (within 10-9 s). (5) For a given metal, there is a frequency of light below which no photoelectrons are emitted. Also, for each frequency f, there is a maximum energy which photoelectrons can have. Let’s add the experimental results to our table: Predict Observe PA2, PI Eelectron Ilight N(e-) Ilight, Ee- independent of Ilight Eelectron (flight)2 Eelectron (flight) Vextinction (flight)2 Vextinction (flight) tescape = “very long” tescape = “instantaneous” no limit to electron KEmax, no Imin to produce e- there is a maximum electron KE, there is an Imin needed to produce e- Theorists: your comments? Experimentalists: your comments? We have a problem here, don't we? What to do? The experiment (correctly done, of course) always provides guidance for the theorist. Let’s look at the data. These graphs show current vs. retarding voltage for the same metal. Graphs from http://www.chembio.uoguelph.ca/educmat/ chm386/rudiment/tourexp/photelec.htm. (broken link, January 2005?) If you take data for several metals and then plot stopping potential vs. light frequency, you get this: Electrons leave the metal with KE. The stopping potential is proportional to the maximum KE. Graph from http://www.chembio.uoguelph.ca/ educmat/chm386/rudiment/tourexp/photelec.htm. (broken link, January 2005?) Here’s a plot of the maximum photoelectron energy versus frequency of incident light: straight line; y = mx + b remember, we’ll use f, not The plots of Kmax vs. f obey the relationship K max = hf - hf0 , where h is a constant, f is the frequency of the incident light, and f0 is the threshold frequency below which no photoelectrons are emitted. The constant h has the same value for all metals, but f0 depends on the metal. Planck’s constant = h = 6.63x10-34 Js = 4.14x10-15 eVs. It sounds like we’re on to something, doesn’t it, but keep in mind… this is an empirical equation; i.e., it fits the experiment, but we haven't explained anything. Have you heard the term “empirical parameter?” What does it mean? So what is the “real” meaning of the term “empirical parameter?” So we have a theory full of holes (Rayleigh/Jeans)… …and an empirical equation that works only because we’ve thrown in a fudge factor. Who you gonna call for help? Einstein's hypothesis and explanation for the photoelectric effect. Einstein postulated that a beam of light consists of small bundles of energy, called "light quanta" or "photons." The energy of a photon is given by E=hf. An electron can absorb all of a photon's energy or none of it, but nothing in between. Ephoton = hf . Some electrons may acquire enough energy to escape from the illuminated metal surface (the escape energy is called the work function of the surface). Electrons escaping from the metal may or may not use up additional energy in escaping. The maximum energy electrons can leave the metal with is equal to hf minus the work function. Light of frequency f can't give an electron any more energy than hf. Thus, according to Einstein, the empirical equation for the photoelectric effect really says* hf = K max + hf0 , energy you start with energy you leave with energy you use to escape where Kmax is the maximum photoelectric energy and hf0 is the work function energy. The equation is just an expression of conservation of energy; the “big deal” is the idea of the photon. Einstein won the 1921 Nobel Prize for explaining the photoelectric effect. He never won a Nobel Prize for his work in relativity! *This may look like just a rearrangement of our previous equation. The difference is that this equation is part of a testable theory. Huge difference! Einstein brilliantly explained all of the features of the photoelectric effect, but his ideas were so revolutionary in 1905 that they weren’t really accepted until 1916 when Millikan provided conclusive experimental verification. Actually, Millikan viewed Einstein’s explanation of the photoelectric effect as a direct attack on the wave nature of electromagnetic waves, and worked very hard for a decade to prove Einstein wrong. Instead, Millikan proved Einstein right.* *If it’s any consolation, Millikan won the 1923 Nobel Prize for proving Einstein right. Example. Homework Problem 2.11. The maximum wavelength for photoelectric emission in tungsten is 230 nm. What wavelength of light must be used in order for electrons with a maximum energy of 1.5 eV to be ejected? The first step is to interpret the problem. Photons with >230 nm are lower in energy than 230 nm photons. The problem has given you the minimum energy photon required to eject an electron. This minimum energy is equal to the work function: hc φ = hf0 = . λ0 Remember, for an E&M wave, c = f . c = fλ , E&M wave. Now you can use our OSE: hf = K max + hf0 hf = K max hc + λ0 hc hc = K max + λ λ0 hc hc λ K max + λ 0 =1 This is not the only way to solve it. I do suggest you do the algebra first, then plug in numbers at the end. hc λ K max hc + λ0 Plug in the numbers, get the answer! Now we have light (and E&M waves) all figured out. It (light) has all the properties of a wave… …except sometimes it has the properties of a particle.