Latin Word Meaning “Poison”
advertisement

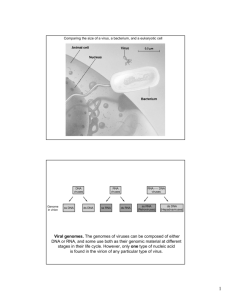
Latin Word Meaning “Poison” Definitions: Viruses are obligate intracellular parasites with a relatively simple life cycle and Viruses are entities whose genomes (nucleic acids) replicate inside living cells using the cellular biosynthetic machinery and cause the synthesis of specialized elements (virions) that can transfer the viral genome to other cells. SOME HISTORY OF VIRUSES: plant case first to be discovered, early-1890’s (TMV) first to be crystallized, 1935 (TMV) first to be reconstituted, 1955 (TMV) animal case discovered, mid-1890’s (FMDV) cell culture and plaque assays 1952 vaccines (polio), 1950’s; gene delivery… bacterial case discovered, 1915 (“intestinal phage”) phage and the origin of molecular biology, 1937 -first EM pictures, 1941 Characteristics of Life • 1. Cells • 2. Grow and maintain their structure by taking up chemicals and energy from the environment • 3. Respond to their external environment • 4. Reproduce and pass on their organization to their offspring • 5. Evolve and Adapt to their environment Viruses are: • 1. Acellular • 2. Obligate intracellular parasites Requires appropriate host cell for almost all metabolism • 3. No ATP generating system • 4. No Ribosomes or means of Protein Synthesis Host range • Spectrum of host cells that a virus can infect • Host range limited by – Type of host – Type of cell • Some viruses only infect: – – – – – plants invertebrates protists fungi bacteria (Bacteriophages) • Egs. • – Measles, polio, smallpox; humans only – West Nile virus; mosquitos, birds, humans Most viruses have a narrow host range Polio virus - nerve cells Adenovirus - cells in upper Respiratory Tract (cold Virus) Host range is determined by Viruses ability to interact with its host cell • Binding Sites match Receptor Sites • Binding Sites - on viral capsid or envelope • Receptor Sites - on host cell membrane Viral Size 20 nm to 1,000 nm .02 u to 1 u Comparing the size of a virus, a bacterium, and an animal cell Virus Bacterium Animal cell Animal cell nucleus 0.25 m Viral Structure SIMPLEST VIRUSES ARE JUST: • 1. Nucleic Acid – DNA or RNA (But never both) • • • • ssDNA ds DNA ss RNA ds RNA 2. Capsid: Protein coat enclosing viral genome Capsomeres: Protein subunits form into regular shapes Rods, icosahedrons, polyhedral head with a tail) • Some Viruses: a protein shell “ capsid” • A. Envelope: cylindrical spherical – derived from the host cell with embedded viral proteins • or some combination of the two shapes – Binding Sites B. Enzymes • without or with a lipid bilayer envelope Capsid Functions • Packaging or Condensation • Protects viral genome (Nucleic Acid) from host endonucleases • Transport Nucleic Acid From Cell to Cell • Provides Specificity for Attachment HELICAL ICOSAHEDRAL CAPSOMER ARRANGEMENT Penton Capsomer (Surrounded by 5) Hexon Capsomer (Surrounded by 6) Electron Micrographs of Helical Nucleocapsids of RNA Viruses A. Measles B. Influenza C. VSV Viral Morphology 1. Helical Viral Morphology 2. Polyhedral icosahedral Viral Morphology 3. Enveloped A. Enveloped Helical B. Enveloped Polyhedral Viral Morphology 4. Complex Capsomere of capsid RNA Capsomere Membranous envelope DNA Head Capsid Tail sheath RNA DNA Tail fiber Glycoprotein Glycoprotein 18 250 mm 70–90 nm (diameter) 20 nm (a) Tobacco mosaic virus 50 nm (b) Adenoviruses 80–200 nm (diameter) 50 nm (c) Influenza viruses 80 225 nm 50 nm (d) Bacteriophage T4 Growing Viruses • 1. Bacteriophages – Lawn of Bacteria on a Spread Plate – Add Bacteriophages – Infection will result in “Plaques” • Clear zones on plate Growing Viruses • Animal Viruses – A. Living Animals • mice, rabbits, guinea pigs – B. Chicken Embryos (Eggs) • used to be most common method to grow viruses • Still used to produce many vaccines (Flu Vaccine) – C. Cell Cultures • Most common method to grow viruses today Viroids and Prions • Viroids – Naked RNA (no capsid) – 300 – 400 nucleotides long – Closed, folded, 3-dimensional shape (protect against endonucleases ?) – Plant pathogens – Base sequence similar to introns Prions • Proteinaceous infectious particle • 1982 • Diseases – – – – Scrapie (sheep) Creutzfeldt-Jacob disease (CJD) Kuru (Tribes in New Guinea) Bovine Spongiform Encephalopathy (BSE) • Mad Cow Disease DNA VIRUS GENOMES Single Stranded Double Stranded RNA + or - Segmented Double Stranded Segmented Circular RNA Viruses HELICAL ICOSAHEDRAL ENV. ENV. NON-ENV. SS SS DS SS TOGAVIRIDAE FLAVIVIRIDAE (RETROVIRIDAE) PICORNAVIRIDAE CALICIVIRIDAE REOVIRIDAE RHABDOVIRIDAE PARAMYXOVIRIDAE BUNYAVIRIDAE ARENAVIRIDAE ORTHOMYXOVIRIDAE CORONAVIRIDAE (RETROVIRIDAE) RNA VIRUSES TOGAVIRIDAE FLAVIVIRIDAE BUNYAVIRIDAE PICORNAVIRIDAE REOVIRIDAE RHABDOVIRIDAE ARENAVIRIDAE CORONAVIRIDAE PARAMYXOVIRIDAE ORTHOMYXOVIRIDAE RETROVIRIDAE DNA Viruses ICOSAHEDRAL ENV. DS HERPESVIRIDAE HEPADNAVIRIDAE COMPLEX NON-ENV. SS PARVOVIRIDAE ENV. DS PAPOVAVIRIDAE ADENOVIRIDAE DS POXVIRIDAE Virus attachment to the cell Viral Replication (reproduction) • • • • Genome enters host cell Host enzymes and components used to make new viral parts Viral genome and capsid spontaneously self-assemble Completed, infectious viruses exit the cell • Bacteriophage – 1. Lytic Cycle – 2. Lysogenic Cycle One mechanism for viral entry into the cell: Receptor-mediated endocytosis One Step Growth Cycle VIRIONS PER CELL 1000 TOTAL VIRIONS 100 MULTIPLICITY OF INFECTION EXTRA CELLULAR or RELEASED VIRIONS YIELD (BURST SIZE) 10 LATENT PERIOD 1 ECLIPSE VIRIONS PER CELL PERIOD 0.1 1 7 10 15 HOURS AFTER ADSORPTION 20 ATTACHMENT HOST FUNCTIONS PENETRATION UNCOATING Transcription Translation VIRAL LIFE CYCLE REPLICATION ASSEMBLY (MATURATION) RELEASE Simplified viral reproductive cycle Entry into cell and uncoating of DNA DNA VIRUS Capsid Transcription Replication HOST CELL Viral DNA mRNA Viral DNA Capsid proteins Self-assembly of new virus particles and their exit from cell Lytic vs lysogenic • Lytic cycle (virulent phage) Assembly (Maturation): viral particles are assembled Release of virus burst and kills host cell (Lysis) • Lysogenic cycle (temperate phage) – Viral DNA integrates into host genome • 1 phage protein prevents transcription of other phage genes – Can be transmitted to daughter cells – Can initiate lytic cycle in response to environmental signal Bacterial viruses Bacteriophages (Phages) • Viruses that infect bacteria • Bacteriophages cannot reproduce and survive on their own, must take over host cell • Fundamentally important microbes – Controlling bacteria populations and energy cycling – Gene transfer and shuffling in the environment – Tools for molecular biology and recombinant DNA technology T4 bacteriophage infecting an E. coli cell Applications of Phage Biology • Need for alternative therapies for treating bacterial infections – Resistance exists to every antibiotic we have • • • • • Phages are potent antibacterials Self-replicating (smart drugs?) Narrow specificity so don’t damage the normal flora Resistance not as significant Resurgent interest in the application of phages to agriculture and human health • Used for years in Eastern Europe and Russia Classification of Bacteriophages • • • • • The most important criteria used for classification are phage morphology and nucleic acid properties Flexible tail (lambda) Contractile tail (T4) Morphology – Head shape – Contractile tails – Noncontractile tails – Tailless – Filamentous dsDNA ssDNA Filamentous (fd) ssRNA dsRNA Tailless (SSV-1) Morphology: T4 Head / Capsid DNA Sheath (Tail) Tail fibres Base plate dsDNA Phage Life Cycle • Vast majority of phages are dsDNA • Two life styles – Lytic (e.g. T4) – Lysogenic (e.g. Lambda) Lytic Life Cycle - 1 • Adsorption to the host cell and penetration – Specificity of phage infection • ~10 phages for every type of bacteria – Phage attach to specific receptor sites on bacterial surface • Proteins • Lipopolysaccharides • Teichoic acids and cell wall components • Carbohydrates • Sex pilus – Phages then inject DNA into the cell • Tail contraction (T4) • Injection (PRD1) • Unknown mechanisms Phage DNA Bacterial DNA Lytic Life Cycle - 2 • Synthesis of phage nucleic acids and proteins – mRNA molecules transcribed early in the infection are synthesized using host RNA polymerase (1 min) • Make viral enzymes required to take over the host cell – Degradation of host DNA (3 min) – Transcription of viral genes (5-9 min) • Phage DNA is replicated (5 min) – Phage DNA sometimes modified protect the phage DNA from host enzymes (restriction endonucleases) that would degrade the viral DNA • The assembly of phage particles – Phage mRNA directs the synthesis of capsid proteins and other proteins involved in assembly and release of the virus (12 min) – DNA packaged into the head (13 min) – Phage particles assembled (15 min) Lytic Life Cycle - 3 • Release of phage particles (22 min – 300 new phage particles) – Many phages lyse their host by damaging the cell membrane and cell wall • Holin – enzyme which destabilizes the host cell membrane (pokes holes) • Lysin – phage enzyme which breaks host cell wall (lyses host bacteria) Lytic Life Cycle - Summary • • • • • Adsorption to the host cell and penetration – Viruses attach to specific receptor sites (proteins, lipopolysaccharides, teichoic acids, etc.) on the host cell – Many viruses inject DNA into the host cell, leaving an empty capsid outside Synthesis of phage nucleic acids and proteins – mRNA molecules transcribed early in the infection (early mRNA) are synthesized using host RNA polymerase; • make viral enzymes required to take over the host cell – Transcription of viral genes follows Phage DNA is replicated – Phage DNA sometimes modified protect the phage DNA from host enzymes that would degrade the viral DNA The assembly of phage particles – Phage mRNA directs the synthesis of capsid proteins and other proteins involved in assembly and release of the virus – Phage pieces assembled – DNA packaged into the head Release of phage particles – Many phages lyse their host by damaging the cell wall or the cytoplasmic membrane – A few phages (e.g., filamentous phages) are released without lysing the host cell – secreted instead The lytic cycle of phage T4, a virulent phage 1 Attachment. The T4 phage uses its tail fibers to bind to specific receptor sites on the outer surface of an E. coli cell. 5 Release. The phage directs production of an enzyme that damages the bacterial cell wall, allowing fluid to enter. The cell swells and finally bursts, releasing 100 to 200 phage particles. 2 Entry of phage DNA and degradation of host DNA. The sheath of the tail contracts, injecting the phage DNA into the cell and leaving an empty capsid outside. The cell’s DNA is hydrolyzed. Phage assembly 4 Assembly. Three separate sets of proteins self-assemble to form phage heads, tails, and tail fibers. The phage genome is packaged inside the capsid as the head forms. Head Tails Tail fibers 3 Synthesis of viral genomes and proteins. The phage DNA directs production of phage proteins and copies of the phage genome by host enzymes, using components within the cell. OVERVIEW OF VIRUS LIFE CYCLE Adsorption (cell Surface) Penetration Uncoating (Cyto. or Nuc.) Eclipse Begins Biosynthetic Period (Cyto. and/or Nuc.) Genome Replication Assembly (Cyto. or Nuc.) Eclipse Ends Release From Cell Infection of Other Cells The lytic and lysogenic cycles of phage , a temperate phage Phage DNA The phage attaches to a host cell and injects its DNA. Many cell divisions produce a large population of bacteria infected with the prophage. Phage DNA circularizes Phage Occasionally, a prophage exits the bacterial chromosome, initiating a lytic cycle. Bacterial chromosome Lytic cycle Lysogenic cycle Certain factors determine whether The cell lyses, releasing phages. Lytic cycle is induced New phage DNA and proteins are synthesized and assembled into phages. or Lysogenic cycle is entered Prophage Phage DNA integrates into the bacterial chromosome, becoming a prophage. The bacterium reproduces normally, copying the prophage and transmitting it to daughter cells. Measuring Phage Number – Plaque Assays • Plaque assay – method for enumerating the number of phage particles in a sample; results are given in plaque forming units (PFU) agar plate (1.5%) Mix phage and bacteria The Polymerase Chain Reaction (PCR) • • • • PCR is used to synthesize large quantities of a specific DNA fragment in vitro (in a test tube) Synthetic DNA molecules with sequences identical the target sequence are created during the reaction Replication is carried out in successive heatingcooling cycles using a heat-stable DNA polymerase from a thermophilic bacteria PCR has proven valuable in molecular biology, medicine (e.g., PCR-based diagnostic tests) and in biotechnology (e.g., use of DNA fingerprinting in forensic science) PCR Human viruses PICORNAVIRIDAE IMPORTANT ASPECTS 1. Small RNA viruses 2. Cause variety of human diseases, including poliomyelitis and common cold 3. Exhibit post-translational maturation of viral proteins PICORNAVIRIDAE GENERA Characteristic Members Enterovirus Poliovirus, coxsackievirus Cardiovirus Encephalomyocarditis virus Theiler’s virus Rhinovirus Rhinovirus Aphthovirus Non-human viruses Hepatovirus Hepatitis A virus Picornaviruses PICORNAVIRIDAE SIZE (nm): 27 ENVELOPED: No CAPSID SYMM: NUCLEIC ACID: CLASS: Icosahedral RNA IV FORM: ss+; 3' poly(A) 5' protein (VPg) SEG: 1 GENES: GENOME SIZE: 6–8 7 – 8 kb MEMBERS poliovirus, rhinovirus, coxsackievirus, hepatitis A virus (+) Strand Viral RNA 5 3 HN 2 POLYPROTEIN P2 P1 1AB 1A 1C 1B COOH P3 1D Production & Maturation of Polyprotein REPLICATION OF GENOME OF PLUS STRAND RNA VIRUS (+) 5 (+) 5 3 Parental (+) Strand 3 Protein Synthesis 3 Minus Strand Synthesis (Stage 1) 5 Plus Strand Synthesis (Stage 2) (+) 5 (-) (-) (-) 3 (+) (+) Progeny (+) Strands Picornavirus RNA Synthesis Strategy Enterovirus (poliovirus) Morphogenesis HUMAN POLIOMYELITIS Small Intestine Bloodstream Day 2 Invasion Multiplication (Final excretion in feces through large intestine) CNS EFFECT PARALYSIS Day 10 Invasion Multiplication Intraneural Spread Day 6 Primary Viremia Viral Multiplication SALK POLIO VACCINES SABIN Inactivated Virus TYPE Attenuated Live Virus Intramuscular Injection ADMINISTRATION Oral 3 Univalent Injections Lifelong DOSAGE 5 Multivalent Doses IMMUNITY Lifelong IgG DIFFERENCE IgG, IgA Free From Vaccine Caused Infections SAFETY Small Number of Infections Caused By Vaccine Hepatitis A Virus Transmission • Close personal contact (e.g., household contact, sex contact, child day care centers) • Contaminated food, water (e.g., infected food handlers, raw shellfish) • Blood exposure (rare) (e.g., injecting drug use, transfusion) Geographic Distribution of HAV Infection Anti-HAV Prevalence High Intermediate Low Very Low Caliciviruses Togaviruses TOGAVIRIDAE SIZE (nm): ENVELOPED: CAPSID SYMM: NUCLEIC ACID: CLASS: 70 Yes Icosahedral RNA IV SEG: ss+; 5' cap 3' poly(A) 1 GENES: GENOME SIZE: 7-8 10 – 12 kb FORM: MEMBERS Sindbis virus, Semliki Forest virus, WEE, EEE, rubella virus Togavirus protein expression Togavirus RNA Synthesis Strategy TOGAVIRUSES EARLY LATE RNA Released from nucleocapsid 42s RNA (genomic) 26s RNA (subgenomic) NON-STRUCTURAL PROTEINS STRUCTURAL (or VIRION) PROTEINS Flaviviruses FLAVIVIRIDAE SIZE (nm): ENVELOPED: CAPSID SYMM: NUCLEIC ACID: CLASS: 45 - 60 Yes Icosahedral RNA IV FORM: ss+ SEG: 1 GENES: 8 - 10 GENOME SIZE: 10 - 11 kb MEMBERS Yellow fever virus, Dengue virus, West Nile virus St. Louis encephalitis virus, Japanese encephalitis virus Flavivirus RNA Synthesis Strategy Key Clinical Facts about West Nile Virus in North America West Nile virus infection is a mosquito-borne infection with a rapidly expanding geographic distribution. One in 5 infected individuals develops mild febrile illness; 1 in 150 develops meningitis, encephalitis, or both. Advanced age is by far the greatest risk factor for severe neurologic disease, long-term morbidity, and death. Presence of West Nile virus-infected birds, onset of meningitis or encephalitis in late summer or early fall, and profound muscle weakness provide important diagnostic clues. IgM antibody capture ELISA testing of cerebrospinal fluid or serum is the most efficient diagnostic method; testing is available through state and local health departments; false-positive results may occur after other flavivirus infections or vaccinations. Rapid reporting of possible cases to health departments is essential to guide public health control efforts. From: Petersen and Marfin (2002) Ann. Intern. Med. 137:173-179 Transmission cycle of West Nile virus From: Petersen and Marfin (2002) Ann. Intern. Med. 137:173-179 Culex mosquito laying eggs Symptoms of West Nile Virus Reported among Hospitalized Patients during Outbreaks in New York State (1999), Romania (1996), and Israel (2000) Petersen, L. R. et. al. Ann Intern Med 2002;137:173-179 Schematic of virologic and serologic tests in West Nile virus encephalitis Gea-Banacloche, J. et. al. Ann Intern Med 2004;140:545-553 West Nile Virus Disease in the United States, 1999-2003 Gea-Banacloche, J. et. al. Ann Intern Med 2004;140:545-553 2003 West Nile Virus Activity in the United States 2004 West Nile Virus Activity in the United States (reported to CDC as of October 26, 2004) Electron micrograph of West Nile virus isolated from brain tissue from a crow found in New York Magnification: 65,625 X Features of Hepatitis C Virus Infection Incubation period Acute illness (jaundice) Case fatality rate Chronic infection Chronic hepatitis Cirrhosis Mortality from CLD Average 6-7 weeks Range 2-26 weeks Mild (<20%) Low 75%-85% 70% (most asx) 10%-20% 1%-5% Hepatitis C Virus Infection, United States New infections (cases)/year 1985-89 1998 242,000 (42,000) 40,000 (6,500) Deaths from acute liver failure Rare Persons ever infected (1.8%) 3.9 million (3.1-4.8)* Persons with chronic infection 2.7 million (2.4-3.0)* Of chronic liver disease - HCV-related 40% - 60% Deaths from chronic disease/year 8,000-10,000 . %95*Confidence Interval Transmission of HCV • Percutaneous – Injecting drug use – Clotting factors before viral inactivation – Transfusion, transplant from infected donor – Therapeutic (contaminated equipment, unsafe injection practices) – Occupational (needlestick) • Permucosal – Perinatal – Sexual ARBOVIRUSES Group of Viruses Containing Several Families of ARthropod-BOrne And Related Viruses ARBOVIRUS Included Families TRANSMITTED By MOSQUITOES Or TICKS Togaviridae Flaviviridae Bunyaviridae Reoviridae CORONAVIRIDAE SIZE (nm): ENVELOPED: 80 - 120 Yes CAPSID SYMM: Helix NUCLEIC ACID: CLASS: RNA FORM: SEG: GENES: GENOME SIZE: IV ss+; 5' cap 3' poly(A) 1 7 27 - 32 kb MEMBERS human respiratory coronavirus, SARS-CoV mouse hepatitis virus, infectious bronchitis virus (avian) Coronavirus Replication Scheme Electron micrograph of SARS-CoV virion Enhanced electron micrograph of SARS-CoV virions Updated Interim U.S. Case Definition for Severe Acute Respiratory Syndrome (SARS) CDC 7/03 Clinical Criteria Asymptomatic or mild respiratory illness Moderate respiratory illness Temperature of >100.4°F (>38°C)*, and One or more clinical findings of respiratory illness (e.g., cough, shortness of breath, difficulty breathing, or hypoxia). Severe respiratory illness Temperature of >100.4°F (>38°C)*, and One or more clinical findings of respiratory illness (e.g., cough, shortness of breath, difficulty breathing, or hypoxia), and radiographic evidence of pneumonia, or respiratory distress syndrome, or autopsy findings consistent with pneumonia or respiratory distress syndrome without an identifiable cause SARS Epidemiologic Criteria Travel (including transit in an airport) within 10 days of onset of symptoms to an area with current or previously documented or suspected community transmission of SARS [China (Mainland); Hong Kong; Hanoi, Vietnam; Singapore; Toronto, Canada; Taiwan], or § Close contact within 10 days of onset of symptoms with a person known or suspected to have SARS. § Close contact is defined as having cared for or lived with a person known to have SARS or having a high likelihood of direct contact with respiratory secretions and/or body fluids of a patient known to have SARS. Examples of close contact include kissing or embracing, sharing eating or drinking utensils, close conversation (<3 feet), physical examination, and any other direct physical contact between persons. Close contact does not include activities such as walking by a person or sitting across a waiting room or office for a brief period of time. SARS Laboratory Criteria Confirmed Detection of antibody to SARS-associated coronavirus (SARS-CoV) in a serum sample, or Detection of SARS-CoV RNA by RT-PCR confirmed by a second PCR assay, by using a second aliquot of the specimen and a different set of PCR primers, or Isolation of SARS-CoV. Negative Absence of antibody to SARS-CoV in a convalescent–phase serum sample obtained >28 days after symptom onset.** Undetermined Laboratory testing either not performed or incomplete. SARS Cases in the United States, Spring 2003 Type of Case No. CoV+* CoV-* Pending Probable 74 8 38 28 Suspect 344 0 169 175 *Based on presence of absence of SARS antibody at > 28 days Lessons Learned: Key Epidemiologic Features • • • • SARS can spread rapidly around the world Healthcare facilities played central role Most cases were spread person-to-person Vast majority of febrile respiratory infections in U.S. were not SARS Positive strand RNA virus comparison Virus family Envelope Polyproteins Subgenomic mRNAs Picornaviruses No Yes No Caliciviruses No Yes No Togaviruses Yes Yes Yes (1) Flaviviruses Yes Yes No Coronaviruses Yes Yes Yes (6 – 7) Examples of cytopathic effects of viral infection of animal cells PLAQUE REDUCTION TEST Used to determine Ab titer to a given virus in a test serum: 1. Use Dilution Of Virus Stock That Yields A Fixed # Of Plaques On Susceptible Monolayers Of Cultured Cells. 2. Mix This Dilution of Virus Stock With Serial Dilutions Of The Test Serum. 3. Plate Out Dilutions On Cell Monolayers. 4. If Ab Present, Will Neutralize Virus And Prevent Plaque Formation. 5. Quantitate Ab Titer As The Highest Dilution That Will Neutralize The Plaque Formation Of the Specific Virus Dilution Being Used. Particle-to-plaque-forming-unit ratios of some animal viruses Plant Viruses Infection by tobacco mosaic virus (TMV)