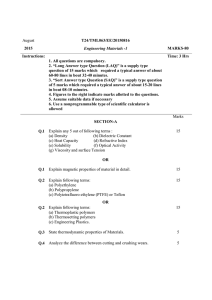
FME 2014
advertisement
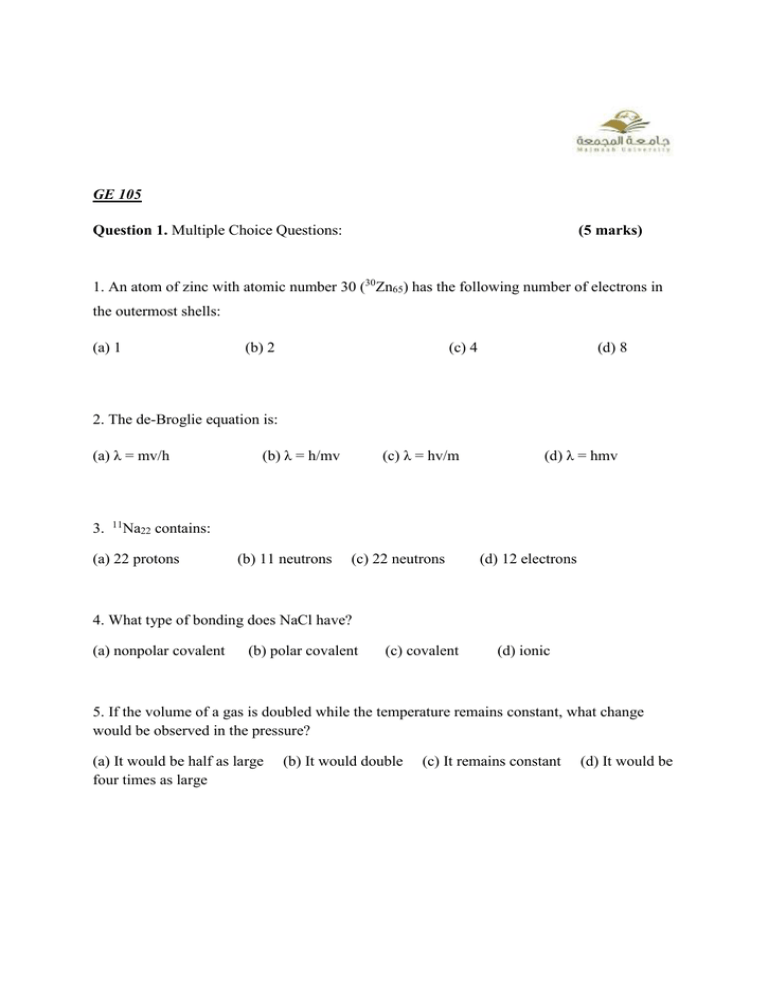
GE 105 Question 1. Multiple Choice Questions: (5 marks) 1. An atom of zinc with atomic number 30 (30Zn65) has the following number of electrons in the outermost shells: (a) 1 (b) 2 (c) 4 (d) 8 2. The de-Broglie equation is: (a) λ = mv/h 3. 11 (b) λ = h/mv (c) λ = hv/m (d) λ = hmv Na22 contains: (a) 22 protons (b) 11 neutrons (c) 22 neutrons (d) 12 electrons 4. What type of bonding does NaCl have? (a) nonpolar covalent (b) polar covalent (c) covalent (d) ionic 5. If the volume of a gas is doubled while the temperature remains constant, what change would be observed in the pressure? (a) It would be half as large four times as large (b) It would double (c) It remains constant (d) It would be Question 2. True or False questions (5 marks) 1. Uncertainity principle has been given by Pauli 2. Magnetic quantum number (m) has the value ranging from –l to +l 3. Boyle’s law can be written as P1V1 = P2V2. 4. Polymers are macromolecules (large molecules) 5. An ionic bond is the attraction between a positive ion and a negative ion. Question 3. Short Questions 1. Classify polymers on the basis of source. (5 marks) 2. An electron is in a 4f orbital. What are the values of the quantum numbers n, l, and m? 3. Write down the electronic configuration of 29 Cu63, 15 P30. 4. Explain Avagadro’s law. 5. What is covalent bonding? Give three examples. Question 4. Numerical problems: 1. At what temperature would 2.60 moles of N2 gas have a pressure of 1.75 atm and in a 20.0 L tank? (2 marks) 2. A container holds 500 mL of CO2 at 20° C and 1 atm. What will be the volume of the CO2 if the pressure is increased to 2 atm? (2 marks) 3. Calculate the wavelength associated with an electron (mass 9.1 × 10-31 kg) moving with a velocity of 1013 m sec-1 (h= 6.6 × 10-34 kg m2sec-1). (1 mark)