Conflicts of Interest for the Research Investigator
advertisement

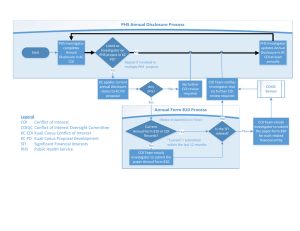
Conflicts of Interest for the Research Investigator Camille A. McWhirter, J.D. Director, Research Compliance USF Health Learning Objectives Generally: Define a conflict of interest (COI) Recognize different types of “self-interest” that can create a conflict of interest Understand what types of “self-interest” are monitored in the research context and why Learning Objectives (cont) Regulatory Context: Understand the difference between the state’s interest in regulating COI and the federal government’s interest in regulating COI Identify the three federal government agencies who have rules regulating COI in research Understand when each agency’s rules apply Learning Objectives (cont) Practical Application: Identify the types of financial interests that must be reported prior to engaging in research Know when to report financial interests and to whom Understand how a COI Committee evaluates financial interests Be familiar with the types of management controls available to manage conflicts of interest in research “Working” Definitions Conflict of Interest A conflict of interest exists when an individual’s professional or ethical obligations might be compromised by self-interest. Conflicts of Interest and Commitment w/ University Employment (Excessive time spent on external endeavors, use of USF resources/name/students in furtherance of private interests, waiver of USF IP rights in private agreements) Conflicts of Interest in Research (Personal financial interests that may bias the design, conduct or reporting of USF Research) General Conflicts of Interest (Nepotism, Doing Business w/USF in Private Capacity, Board/Committee Membership, Purchasing Decisions) “Working” Definitions Conflict of Interest in Research A conflict of interest in research exists when an investigator’s self-interests have the potential to compromise an investigator's professional judgment and objectivity in the design, conduct or reporting of research. Investigator Any person responsible for the design, conduct or reporting of research. COI = Individual + Self-Interest + An Ethical or Professional Duty COI in Research = Investigator + Self-Interest + Objectivity in the Design/Conduct/Reporting of Research HINT: The management goal is to protect the integrity of the research Hypothetical: COI? Dr. Needbucks of Public University invented a drug to prevent Alzheimer’s disease. DevicesRUs, wants to sponsor a study with Dr. N as the PI to develop and test a device that will deliver Dr. N’s drug directly to the brain. Dr. N’s spouse is the president of Lab Solutions, Inc. that manufactures lab supplies and equipment. Dr. N and her staff purchase all of their basic supplies and equipment from Lab Solutions. Ask: Investigator? Yes. The individual is an investigator on a research study. Self-Interest? Yes, financial interest of spouse in a company that sells research products. What is the professional/ethical obligation that is compromised? The duty potentially compromised by this self-interest is the duty of public stewardship--to refrain from using one’s public position to enhance one’s private interests. Conclusion: This is a conflict of interest in a research setting, but not a “COI in Research” as we have defined it because the investigator’s self-dealing, while perhaps a conflict with her public position, does not compromise the integrity of the research results. What if during the course of the study… Dr. Needbucks purchases $30,000 worth of stock in DevicesRUs? Repeat the analysis: Investigator? Yes. The individual is an investigator on a research study. Self-Interest? Yes, Dr. N’s financial investment in the sponsor/manufacturer of the device being tested. What is the professional/ethical obligation that is compromised? The duty potentially compromised by this self-interest is the duty of objectivity in the design, conduct, or reporting of the research study. Conclusion: This is a “COI in Research” as we have defined it. Note that there may be more than one type of conflict of interest at work in the research context—multiple avenues of self-interest and multiple professional or ethical duties. Types of Self-Interest Self-interest can manifest in the form of A financial benefit Enhanced reputation Personal relationships Professional relationships Other interests (political, religious, intellectual) What type of self-interest is represented by these examples? Dr. Warren is an investigator for a study testing a new medical device. Dr. Snyder’s brother is one of the owners of the company developing the device. (Personal Relationship) Dr. Mercer sits on the IACUC and reviews a study about cloning. Dr. Mercer thinks cloning is immoral and unethical. (Personal Belief) What type of self-interest is represented by these examples? Dr. O’Connor owns significant stock in a biotech company. The company approaches him about conducting research on the viability of a new genetics test. (Financial Interest) Dr. Tate is the co-founder of a new method of behavioral therapy for which he has received worldwide recognition. He is approached about conducting a study for another form of therapy which, if effective, would render his form of therapy obsolete. (Reputation ) What type of self-interest is represented by these examples? Dr. Markham sits on the board of directors of Company X. Company X is developing a new piece of equipment and they ask Dr. Markham to conduct a study on the safety and efficacy of the equipment. (Professional Relationship) State Regulations (Code of Conduct of Public Officers and Employees) The state regulations only address general COI of public officers and employees and are designed to prohibit and/or manage conflicts between a public employee’s duties to the state employer and the employee’s personal interests Example: A public employee acting in a private capacity, may not sell goods to his or her own agency. Example: A public employee may not have an ongoing or regularly recurring conflict with his or her public employment (catch-all provision). What interests are the state COI regulations designed to protect? Federal Regulations (NSF/PHS and Food & Drug Administration) The federal regulations only address self-interest (i.e., secondary interests) in the research context. Example: Universities receiving federal funds to conduct research must have policies to manage bias in the design, conduct or reporting of research funded with federal dollars (PHS/NSF). Example: A sponsor of a new drug/device must certify that COI of investigators in studies supporting the FDA approval of the drug/device either does not exist or is adequately managed. What interests are the federal regulations designed to protect? Federal Regulations The federal regulations only address one type of “self-interest”: FINANCIAL INTERESTS (Why?) Federal Regulations Food and Drug Administration Applies in research involving a drug, device or biologic. Requirements are directed at the sponsor Requires disclosure at the time of application for approval of a drug device or biologic. Evaluates the impact of the researcher’s financial interests on the reliability of the study. Federal Regulations Public Health Service (PHS) & National Science Foundation (NSF) Requirements are directed at the institutions receiving grants from these agencies Requires institutions to develop a COI policy and program that meets certain minimum requirements One requirement is that the institution must require investigators to disclose significant financial interests in the research Deliberately vague to permit flexibility in the administration of policy PHS and NSF regulations were developed cooperatively to make the regulations consistent. PHS/NSF COI Regulations Investigator Responsibilities: Must report any significant financial interests (including those of spouse and dependent children) to a designated institutional official. Reportable Financial Interests USF policy requires investigators to report not only “significant financial interests”, but: Anything of monetary value, or a potential value that cannot readily be determined, including salary consulting fees honoraria A proprietary interest in the research project, including a patent trademark copyright licensing agreement Reportable Financial Interests (con’t) A position as director, officer, partner, trustee, or member of board of directors of any entity related to the research; A consulting, advisory, employment, ownership/equity or any other interest or relationship in any entity related to the research (including interests in a non-publicly traded corporation of indeterminate value); Any other financial interest or external commitment that the Investigator believes may interfere with his or her ability to protect human research participants There is no minimum amount below which an interest is exempt from reporting! EXCEPTIONS: The following financial interests and commitments are generally recognized as NOT relating to or not being impacted by the outcome of the research and therefore do NOT need to be disclosed: Salary, royalties or other remuneration received from USF or the USFPG. Receipt of royalties for any published scholarly works and other writings. Income from seminars, lectures, or teaching engagements sponsored by public or nonprofit entities. NOTE: All honoraria received from commercial entities must be disclosed. EXCEPTIONS (con’t): Income from service on advisory committees or review panels for public or nonprofit entities. Interests in commercial enterprises on the part of an Investigator which commercial enterprises are in no way related to the Investigator’s professional role and/or obligations. Any “arms length” financial interest (i.e., those that occur through participation in a mutual fund or employer retirement plans), where the participant has no control over the investment decision Income from speaking engagements sponsored by government or non-profit Income from advisory boards of government or non-profit Conflict of Interest Committee USF has a Conflict of Interest Committee (COIC) to evaluate, manage COI in research, and to ensure that the institution’s COI policies and procedures and applicable COI regulations are met. General Principles in Dealing with COI in Research COI is virtually unavoidable. COI does not preclude participation of an investigator in a project. Must have a culture of honest and full disclosure so that steps can be taken to manage conflicts effectively Not all COI can be managed. Disclosure Process for Financial Interests in Research at USF Opportunities to Identify Financial Interests in Research DSR Internal Form Question on COI IRB Application Question on COI DSR Annual Reminder Form Education & Word of Mouth for non-sponsored, non-human subjects studies All Roads lead to the Financial Relationships Disclosure Form When should financial interests be reported on the FRDF? Upon Initial Submission of Research Proposal to DSR (for sponsored research) or to the Investigator’s Department Chair or Committee (for nonsponsored research) prior to engaging in the research. Upon Joining a Research Project. must submit the FRDF within 60 days of beginning work on the research. When should financial interests be reported on the FRDF? (con’t) Upon Acquisition of Any New Reportable Interests. Investigators who have previously submitted an FRDF but who acquire new Reportable Financial Interests or otherwise have a change in the status of such interests must submit a new FRDF within 60 days of the acquisition or change in interest. Annually upon receiving a request from DSR to certify the status of the Investigator’s Reportable Financial Interests Instructions for completion of such annual disclosure forms/certifications will be included with the request from DSR. Disclosure Process at USF (con’t) Completed FRDF Submitted to DSR (all sponsored studies); or USF Health COI Committee (non-sponsored studies) DSR routes FRDF to USF Health COI Committee for studies involving USF Health employees or human subjects CONFLICT OF INTEREST IN RESEARCH: DISCLOSURE PROCESS Research means a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge. STOP. This process does not apply to you. NO START Do you have a financial interest or external commitment that relates to or could be impacted by the outcome of current or proposed research? YES Financial Relationships Disclosure Form Complete a Financial Relationships Disclosure Form (FRDF) GO TO PAGE 2 Financial Interest or External Commitment means Anything of monetary value, or a potential value that cannot readily be determined, a proprietary interest in the Research Project, including a patent, trademark, copyright, licensing agreement or other intellectual property interest in the test article or method; a position as director, officer, partner, trustee, or member of board of directors of any entity Related to the Research; a consulting, advisory, employment, ownership/equity or any other interest or relationship in any entity Related to the Research (including interests in a non-publicly traded corporation the value of which interests cannot be readily determined through reference to public prices); or any other financial interest or external commitment that the Investigator believes may interfere with his or her ability to protect human research participants. Human subject means a living individual about whom an investigator conducting research obtains (1) data through intervention or interaction with the individual or (2) identifiable private information. Does the research involve human subjects? NO FROM PAGE 1 Is this research sponsored? YES Submit form to Division of Sponsored Research (Liz O’Connell ADM 200) NO YES Are you affiliated with USF Health? YES Submit form to USF Health Conflict of Interest (COI) Administrator (Camille McWhirter MDC 2) YES NO Submit form to USF Conflict of Interest in Research (CoIRC) Committee (Vinita Witanachchi MDC 35) STOP STOP NO Does the research involve human subjects? Review Process for Financial Interests in Research at USF (con’t) COI Administrator develops management plan in consultation with interested person COI Administrator may approve without Committee review if certain conditions are met (BUT not for human subjects research) COI Committee Reviews FRDF and Management Plan Notification to Interested Person DSR IRB (if USF IRB is IRB of record) CONFLICT OF INTEREST IN RESEARCH: REVIEW PROCESS NON-SPONSORED, NO HUMAN SUBJECTS, NOT A USF HEALTH EMPLOYEE Submit form to USF Conflict of Interest in Research (CoIRC) Committee (Vinita Witanachchi MDC 35) ALL SPONSORED RESEARCH Submit form to Division of Sponsored Research (Liz O’Connell ADM 200) NONSPONSORED USF HEALTH OR HUMAN SUBJECTS Submit form to USF Health COI Committee (Camille McWhirter MDC 02) USF CoIRC or Administrative Review USF Health COI Committee or Administrative Review Notify investigator of COI determination and forward approval memo and management plan (if any) to DSR, if applicable Notify investigator of COI determination and forward approval memo and management plan (if any) to IRB and DSR, if applicable STOP STOP COI Committee Review MOTIVATION How much incentive does the INV have to bias the design, conduct or reporting of the research? OPPORTUNITY Is the INV in a position to bias the design, conduct or reporting of the research? Possible Steps to Manage COI Elimination of the conflicting interest (e.g., divestiture of financial interest or removal from project) Substitution of non-interested personnel on the project Public disclosure of the financial interests (journals, research subjects, collaborators, etc.) Monitoring of research by independent reviewers Look for inherent management controls (e.g., the research has objective endpoints that are not subject to manipulation by the investigator) What happens next? Once an approved management plan or disclosure is in place, the investigator’s responsibility is to adhere to the terms of the management plan; and submit a new disclosure upon any change in the financial interests upon which the approved management plan or disclosure is based within 60 days of the change in interest. Why Should an Investigator Report Financial Interests? Allows the interest to be evaluated and managed by knowledgeable individuals Promotes public trust and reduction of liability for investigator and institution Triggers institutional processes that safeguard human research participants Reporting and management of financial interests in research is required by USF policy and federal regulations--compliance is necessary to continue receiving federal funding