Sustaining Microbiology Gram Stain Turnaround Time
advertisement

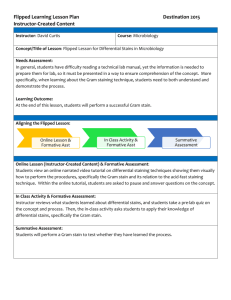
PROJECT NAME: Sustaining Microbiology Gram Stain Turnaround Time Institution: The University of Texas M. D. Anderson Cancer Center Primary Author: Ron Phipps Secondary Author: Joe Yarsa, Pramila Sood Project Category: (Sustain) Choose most appropriate category: 1) Patient Safety, 2) Patient Centered Care, 3) Timeliness, 4) Efficiency, 5) Effectiveness, 6) CS & E Projects Implemented at a New Site, 7) General Quality Improvements or 8)Sustained CS & E Projects Overview: Gram Stains are performed in the Microbiology Lab. This project involves the team of personnel in Microbiology who process, analyze and provide test results for the gram stains. Approximately 250 gram stains are performed each week. Gram stains are performed on body fluid or biopsy when infection is suspected, and is especially important when infection would make an important difference in the patient's treatment and prognosis. The gram stain result is the only report that is available to the physician prior to the culture results, which are usually available a minimum of 24 hours after specimen inoculation. By issuing the gram stain results in a timely manner, the Microbiology Lab is contributing to a more rapid physician response in treating the patients. Therefore, this project contributes to improving overall patient care with the improved gram stain TAT. This project is strategically aligned with the institutional patient care goal of: Strategy 1.2 - We will increase the quality, safety and value of our clinical care. Aim Statement (max points 150): This project focused on the sustainability of a project completed in 2009. The 2008 baseline measures for that project were: 44% of the Routine Gram Stains within 3 hours of receipt in the lab 43% of STAT Gram Stains within 1 hour of receipt in the lab The aggressive aim of that project was to achieve 95% of STAT grams stains within 1 hour and 80% of Routine Gram Stains within 3 hours. The aim of this project was to sustain or improve upon the results achieved at the end of the previous project, which were: 83% of Routine Gram stains within 3 hours of receipt in the lab (mean = 99.9 minutes) 76% of STAT Gram Stains within 60 minutes of receipt in the lab (mean = 55.1 minutes Measures of Success: These are measured from raw data extracted from the Laboratory Information Systems of gram stain tests from all patients. The project scope excludes tests received on Weekends and tests received after 11pm and before 6am on weekdays. The control phase was staged separately from the previous project. The time points are the actual times the LIS records when the specimen is logged in as received in the lab and when the results are first available to the physician. Data was measured and analyzed using Minitab statistical software. Stages were used to assess the impact of the changes being implemented on turnaround times. Use of Quality Tools (max points 250): Since this was a sustainability project, many of the quality tools were part of a previous project. The control plan that was developed to ensure sustainability of results throughout the control phase below. Interventions (max points 150 includes points for innovation): Since this was a sustainability project, many of the quality tools used were part of a previous project. The control plan provided for regular reporting of results, the inclusion of the metrics as quality indicators in the Divisional Quality Council meetings, and corrective actions as needed when conditions were noted as out of control. The team also capitalized on what was working well by communicating and documenting the process steps that were yielding good results. Results (max points 250): Include all results, using control charts, graphs or tables as appropriate. For Routine Gram Stains: 94.3% were completed during the entire Control phase within 3 hours (vs. baseline of 83%) 94.75% were completed within 1 hour for 2012 YTD Jan - June. T-test determined a difference in the mean of 26.3 minutes between the last stage of the previous project and the control phase (95% confidence interval of 24.6 – 28.0 minutes) with a p-value of 0.000. For Stat Gram Stains: 72.4% were completed during 1 hour for the entire Control phase (vs. baseline of 76%) 78.5% were completed within 1 hour for 2012 YTD Jan - June. T-test determined a difference in the mean of 4.5 minutes between the last stage of the previous project and the control phase (95% confidence interval included zero (-1.5 to +10.5). A 1989 Q-Probes study of more than 400 laboratories found actual median turnaround times were 45 minutes for gram stains. Our actual median turnaround times for STAT gram stains has improved since the original baseline from 67.0 minutes to 44.8 minutes and from 199 minutes to 67 minutes for Routine Gram Stains. Revenue Enhancement /Cost Avoidance / Generalizability (max points 200): It is difficult to quantify a return on investment on this project. However, providing a timely response to physicians for this test is critical to patient care. The # of patients with STAT tests now average 87 per month and the # of patients with Routine tests now average 973 per month. Thus, the impact is on 12,720 patients per year. The mean time difference since the end of the previous project of 24.8 minutes for Routine gram stains translates to a savings of 402 hours of lab wait time per month. While we do not have a firm estimate on soft savings, every dollar of soft savings that you could estimate for this project would yield $4,826 annually. So, if every hour saved is “worth” $20, the annual soft savings would be $96,522. The mean difference since the baseline of the first project on STAT gram stains of 46 minutes translates to a savings of 5.8 hours of lab wait time per month. Several aspects of the project have been and are being adopted in other projects for generalizability. These include the use of a documented control plan, reporting and posting the control phase metric trends in the work area, and the high level of engagement and attention to detail in understanding the aspects of the process that contribute to delays. In other words, we utilized quality tools like FMEA’s where the causes are all “delay focused”, and based on this project’s success, we have been approached other projects in similar fashion. Conclusions and Next Steps: For our next steps, we will continue to utilize our control plan measures. We are also investigating possible enhancements to the layout of the lab and workflow as part of an upcoming renovation. We will also continue to adopt the best practices from this project to other completed and new projects.