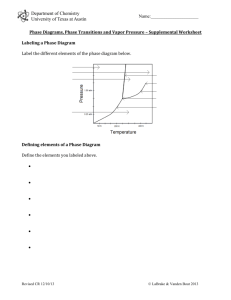
MT 3 practice
advertisement

Midterm 3 1) Which of the following instruments directly measures the pressure of a gas? A) spectrometer B) barometer C) polarimeter D) gas chromatograph 2) Which one of the following is not used to describe the condition of a gas? A) number of moles B) polarity C) temperature D) volume 3) Which process is endothermic? A) Water condenses on the outside of a cold soda can. B) The melted wax hardens after a candle is extinguished. C) Water vapor forms ice crystals in the upper atmosphere. D) Gasoline spilled on the ground evaporates very quickly. 4) Which of the assumptions of the kinetic-molecular theory best explains the observation that a balloon collapses when exposed to liquid nitrogen (which is much colder than a cold winter day!!)? A) Gas molecules move at random with no attractive forces between them. B) The velocity of gas molecules is proportional to their Kelvin temperature. C) The amount of space occupied by a gas is much greater than the space occupied by the actual gas molecules. D) In collisions with the walls of the container or with other molecules, energy is conserved. 5) Volume and pressure are ________ proportional. A) directly B) inversely C) all of the above D) none of the above 6) A basketball is inflated to a pressure of 1.50 atm in a 20.0°C garage. What is the pressure of the basketball outside where the temperature is -5.00°C? A) 1.37 atm B) 1.42 atm C) 1.58 atm D) 1.64 atm 7) Which of the following gases has the highest average speed at 400K? A) CO2 B) N2O4 C) SF6 D) UF6 8) A sample of gas has a volume of 135 mL at 0.600 atm. What would be the volume if the pressure is decreased to 0.200 atm while temperature is held constant? A) 45.0 mL B) 101 mL C) 135 mL D) 405 mL 9) A 250. mL sample of gas at 1.00 atm and 20.0°C has the temperature increased to 40.0°C and the volume increased to 500. mL. What is the new pressure? A) 0.374 atm B) 0.468 atm C) 0.534 atm D) 1.87 atm 10) A sample of CO2 gas at 100.°C has a volume of 250. mL at 760. mm Hg. How many moles of CO2 are present? A) 0.00816 mol B) 0.0304 mol C) 6.20 mol D) 8.16 mol 11) What is the pressure in a 1.00 liter container of methane, CH4, that contains 40.0 g of the gas at 25.0°C? A) 5.13 atm B) 61.0 atm C) 82.1 atm D) 979 atm 12) Which of the following compounds exhibits hydrogen bonding? A) CH3Cl B) HI C) H3C-O-CH3 D) NH3 13) Which of the following molecules is non-polar ? A) C2H2 B) H2O C) CH3CH2OH D) H I 14) Which is expected to have the largest dispersion forces? A) C2H6 B) C8H18 C) N2 D) CO2 15) For which of the following phase changes is the sign of ΔS negative? A) boiling of water B) formation of snow from water vapor in clouds C) melting of ice cream D) sublimation of I2 16) The normal boiling point occurs when the A) intermolecular forces within the liquid phase are broken. B) temperature of the pure liquid equals the external temperature. C) vapor pressure of a pure liquid equals an external pressure of one atmosphere. D) vapor pressure of the liquid equals the external pressure. 17) At a high altitude water boils at 95°C instead of 100°C as at sea level because A) the atmospheric pressure is greater. B) the atmospheric pressure is less. C) the climate is cooler. D) the vapor pressure of water is greater. 18) As the temperature of a liquid is lowered, what happens to its vapor pressure? A) Its vapor pressure drops. B) Its vapor pressure rises. C) The vapor pressure may rise or drop depending on the liquid. D) Its vapor pressure is independent of temperature. E) Its vapor pressure first rises then falls. 19) The vapor pressure of a liquid A) decreases with increasing temperature. B) is independent of temperature. C) is equal to one atmosphere at the normal boiling point. D) cannot be measured. 20) Arrange the following in order of increasing boiling point. CH3CH2OH CH3CH2CH3 H3C-O-CH3 CH3CH2NH2 I II III IV A) IV < III < II < I B) II < III < IV < I C) I < IV < III < II D) II < III < I < IV 21) All of the following statements describing solutions are true except A) making a solution involves a physical change. B) solutions are homogeneous. C) the particles in a solution are atomic or molecular in size. D) solutions are colorless. 22) The number of components in a solution is ________. A) at least 2 B) 3 C) 4 D) 5 23) Which statement best explains the meaning of the phrase "like dissolves like"? A) A solvent will easily dissolve a solute of similar mass. B) A solvent and solute with similar intermolecular forces will readily form a solution. C) The only true solutions are formed when water dissolves a non-polar solute. D) The only true solutions are formed when water dissolves a polar solute. 24) The solubility of gases in liquids A) increases as temperature increases and increases as pressure increases. B) decreases as temperature increases and increases as pressure increases. C) decreases as temperature increases and decreases as pressure increases. D) increases as temperature increases and decreases as pressure increases. 25) When a solid dissolves, each molecule is removed from the crystal by interaction with the solvent. This process of surrounding each ion with solvent molecules is called A) dilution. B) solvation. C) electrolysis. D) hemolysis. 26) In most liquid solutions, the component present in the larger amount is called the A) dispersed medium. B) emulsifying agent. C) solute. D) solvent. 27) The rubbing alcohol sold in drug stores often is composed of 70% isopropyl alcohol and 30% water. In this solution A) isopropyl alcohol is the solvent. B) water is the solvent. C) both water and isopropyl alcohol are solvents. D) neither water nor isopropyl alcohol is a solvent. 28) Although there are exceptions, which is most likely to be true for the dissolving of a solid in a liquid? A) ΔHsoln is positive. B) ΔHsoln is negative. C) ΔSsoln is positive. D) ΔSsoln is negative. 29) All of the statements about molarity are correct except A) the abbreviation is M. B) the interpretation of the symbol is "moles of solute per mole of solvent." C) moles = molarity × volume. D) volume = moles/molarity. 30) What is the % (w/v) concentration of a solution containing 12 grams of solute in 400 mL of solution? A) 1.2% B) 3.0% C) 4.0% D) 6.0% 31) How much NaOH is present in a 75.0 mL sample of a 5.00% (w/v) solution? A) 3.75 g B) 5.00 g C) 6.67 g D) 15.0 g 32) What is the molarity of a solution prepared by dissolving 3.50 mol NaCl in enough water to make 1.50 L of solution? A) 0.429 M B) 2.33 M C) 5.25 M D) 87.8 M 33) What is the molarity of a solution prepared by dissolving 1.25 mol AgNO3 in enough water to make 2.00 × 103 mL of solution? A) 1.25 M B) 2.50 M C) 0.625 M D) 0.00250 M 34) How many moles of HCl are present in 75.0 mL of a 0.200 M solution? A) 15.0 mol B) 2.67 mol C) 0.375 mol D) 0.275 mol 35) A 20.0 mL sample of CuSO4 was evaporated to dryness, leaving 0.967 g of residue. What was the molarity of the original solution? A) 48.4 M B) 0.0207 M C) 0.0484 M D) 0.303 M 36) What is the final concentration of a solution prepared by adding water to 50.0 mL of 1.5 M NaOH to make 1.00 L of solution? A) 0.075 M B) 0.030 M C) 1.5 M D) 7.5 M 37) Which one of the following would act like a strong electrolyte in an aqueous solution? A) KNO3 B) CH3OH C) CCl4 D) NH3 38) Considering 1.0 M solutions of each substance, which contains the largest concentration of ions? A) K2SO4 B) FeCl3 C) NaOH D) NH3 39) Which has the highest boiling point? A) 0.1 M Na2SO4 B) 0.1 M glucose, C6H12O6 C) 0.1 M MgCl2 D) 0.1 M Al(NO3)3 40) Red blood cells are placed in a solution and neither hemolysis nor crenation occurs. Therefore the solution is A) hypertonic. B) hypotonic. C) isotonic. D) isotopic.