Technical Supplement 2A.doc
advertisement

Technical Supplement 2A
The Atom Percent Notation and Measuring Spiked Samples
When working with spiked or isotope-enriched samples, it is often necessary to
switch notations, changing from the notation to the AP (atom percent) notation. This
section shows why this change is needed, and highlights some errors that can occur if the
switch is not made. We start by reviewing the relationships between and AP notations.
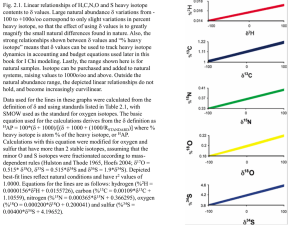
In the range of most natural samples, there is a very close near-linear relationship
between atom percent (or % heavy isotope) and values. Using carbon as an example,
even when substantial amounts of 13C are artificially added up to 10% 13C, this strong
linear relationship persists (Fig. A). However, when the enrichment increases beyond
10%, this simple straight-line relationship starts to break down, and things become very
non-linear at high levels of >90% enrichment (Fig. B). Here the message is that if you
work with both natural abundance and enriched samples in the same study, generally you
should convert your values to “% heavy isotope” or atom percent, where atom % =
100*fractional abundance of the heavy isotope. Recalculating to atom % provides the
same linear scale for all samples, and avoids the curvilinear effects that start to show up
when the isotope data spans large (>1000o/oo) ranges of values. The same linear
scaling applies for values (see end of Section 2.1 for more about using values instead
of values).
We might also note in these examples that no heavy isotope, no 13C in this case,
leads to values of -1000o/oo and not 0o/oo. (This follows from the definition as
follows when substituting zero in the numerator for 13C of the sample. That is, if =
{[(13C/12CSAMPLE)/RSTANDARD] – 1}*1000, substituting “0” for 13C in the numerator means
that 13C/12CSAMPLE = 0, and then = (0/ RSTANDARD] – 1}*1000 = (0-1)*1000 = 1000o/oo). So, it is possible to work with 13C-depleted as well as 13C-enriched materials,
and if you work with very depleted materials, also you may want to keep track of
fractions and atom percent, not .
Before we leave our carbon concerns, let us think harder about measuring
enriched samples with a mass spectrometer. We usually make the carbon isotope
measurements with CO2, a gas that actually has a lot of isotope complexity (Table A).
The stable-isotope versions of the CO2 molecule come in an astonishing array of 17
different isotope combinations, or isotopomers, scattered in 6 mass flavors, from masses
44 to 49 (Table A). Both oxygen and carbon isotopes in the CO2 molecule contribute to
this isotopomer complexity (Table A). Normally, the mass spectrometer has collectors for
the three most abundant isotopomers at masses 44, 45 and 46. Computers monitor these
collectors and make software corrections for oxygen isotope contributions in the final
calculation of 13C values. (These corrections are detailed in the next Technical
Supplement 2B).
With this background, we can ask the question, what happens with enriched
samples? The effect of enrichment, substituting 13C for 12C, is to shift most of the total
signal from mass 44 to mass 45 (Table A). Also, the third-strongest signal is now at mass
47, a mass that mass spectrometer machines normally do not measure (Table A). The
point here is that when dealing with artificially enriched samples, there are sometimes
important changes in measurement collectors and various software calculations that have
to be made to account for all the isotopes, before fractions, atom percent and can be
calculated.
These concerns apply not only for carbon, but also for the other elements such as
nitrogen. For nitrogen, heavy isotope 15N enrichment shifts the main signal from mass 28
to mass 29 and finally to mass 30 at the highest enrichments (Table B, Fig. C). An
important consequence of this is that if normal collectors and software are not adjusted to
include the growing signal at mass 30, and 15N values measured from 29/28 ratios will
underestimate the actual fraction 15N and true 15N value (Fig. D). However, this problem
is relatively minor with low-level (<1% 15N enrichments) typical of many field-ecology
experiments (Fig. E). Technical Supplement 6B in the Chapter 6 folder on this CD
discusses these problems in more detail, and recommends converting results from to
atom % for precise calculations with spiked samples.
If you are measuring only the 29/28 ratios, one nice feature of the theoretical
predictions (Fig. C) is that the 30/28 ratios should vary in concert with the 29/28 ratios.
These results are based on the assumption that the 14N and 15N isotopes are distributed in
a random manner between masses 28, 29 and 30. With this assumption, you can estimate
the true 15N abundances from measured 29/28 ratios without actually measuring 30/28
ratios. This applies even in most tracer work because combustion processes during
sample preparation will randomize isotopes in the N2 that is produced and measured.
Important exceptions for tracer work concern N2O and N2 produced by denitrification and
other processes in 15N-spiked field samples. These two gases are often extracted from
field samples without combustion, and consequently isotopes in these gases usually are
not at equilibrium (Bergsma et al. 2001 and references therein). For N2, estimating mass
30 contributions from equilibrium considerations is too inexact for some N2 enrichment
work, and a better solution is the reconfigure the mass spectrometer to accurately
measure mass 30 (Bergsma et al. 2001). For N2O, current technology favors
measurement of several masses to estimate 15N of both N atoms in the molecule, as well
as the 18O of oxygen (e.g., Popp et al. 2002).
In sum, there is usually a special dimension and mathematics to working with
enriched samples. Also, because it is not always easy to shift collectors and software
associated with the mass spectrometer, it is good to check with your local isotope lab
before you embark on an ambitious program of isotope enrichment (i.e., before you set
off for a good round of “spiking for fun”).
Further Reading
Bergsma, T.T., N.E. Ostrom, M. Emmons and G. P. Robertson. 2001. Measuring
simultaneous fluxes from soil of N2O and N2 in the field using the 15N-gas
“nonequilibrium” technique. Environmental Science and Technology 35: 4307-4312.
Popp, B.N., M.B. Westley, S. Toyoda, T. Miwa, J.E. Dore, N. Yoshida, T.M. Rust, F.J.
Sansone, T.M. Russ, N.E. Ostrom and P.H. Ostrom. 2002. Nitrogen and oxygen
isotopomeric constraints on the origins and sea-to-air flux of N2O in the oligotrophic
subtropical North Pacific gyre. Global Biogeochemical Cycles 16:12-1 to 12-10,
doi:10.1029/2001GB001806.
Figure Legends
Fig. A. Relationships between values and atom %13C. Top: natural abundance range of
values that correspond to about 1.08 to 1.12%13C. Bottom: larger range of atom %
values, 0-10%13C, corresponding to a larger range in values. Note that no 13C (% 13C =
0) corresponds to -1000o/oo value (see text for explanation). Isotope depleted samples
thus have a lower limit (-1000o/oo), but isotope enriched samples have no upper limit, as
evident in the next figures.
Fig. B. Relationships between values and atom %13C, continued. Top: larger range of
atom % values, 0-50%13C. Note the relationship between atom % and starts to become
visibly non-linear at higher enrichments. Bottom: largest range of atom % values 0100%. At high enrichments >90% 13C, and atom % are related in a very non-linear
fashion.
Fig. C. Nitrogen isotopes in N2 gas samples at different 15N enrichments. Modern mass
spectrometers collect three isotopomers (isotope varieties) of N2 that have masses 28
(14N14N), 29 (15N14N and 14N15N) and 30 (15N15N). In natural samples, most N is 14N with
only small amounts of 15N, so most N2 exists as mass 28 with minor amounts as mass 29
and very, very minor amounts as mass 30. However, this can change when scientists start
adding 15N to samples, increasing the fraction that is 15N while simultaneously decreasing
the amount that is 14N. Eventually, when there is no 14N, all N is present as mass 30. At
intermediate mixtures of 14N and 15N, both masses 29 and 30 are important carriers of 15N
and both these masses need to be measured and considered when keeping track of 15N
amounts.
Fig. D. Nitrogen isotopes in N2 gas samples at different 15N enrichments, continued from
previous Fig. C. Top: In most usual measurements, only the mass 28 (14N14N) and mass
29 (15N14N) isotopomers are considered, because the mass 30 (15N15N) contributions are
very, very minor. But at higher 15N enrichments, considering only masses 28 and 29 will
underestimate actual amounts of 15N, as shown in the previous Fig. C. The problem is
that much 15N is in mass 30 for enriched samples, and this is ignored in most
calculations. Bottom: Estimated error in for enriched samples when mass 30 is ignored.
Fig. E. Correction factors (o/oo) for values measured at relatively small 15N
enrichments, <2000o/oo. For example, at 1% 15N (about 1750o/oo 15N), one needs to
add about 8.8o/oo to the normal measured value to obtain the correct 15N value, when
the normal value is calculated from the mass 28 and mass 29 ion beams without
including the mass 30 ion beam.
Table A. Isotope varieties (isotopomers) of CO2 in an unenriched, natural reference
material (left) and in a 99% heavy isotope-enriched sample (right). Note that 13Cenrichment shifts the strongest signal from mass 44 to mass 45 (see arrow below), i.e., for
natural uneriched samples, mass 44 bears most of the carbon isotope signal, while for
99% enriched material, most of the carbon isotope signal appears at mass 45.
% Abundance, 13C-Enriched
% Abundance, Reference (ref)
12
C
13
C
16
O
17
O
18
O
12
98.8944%
1.1056%
99.7553%
0.0385%
0.2062%
C O16O
C
C
16
O
17
O
18
O
13
1%
99%
same as ref
same as ref
same as ref
Mass 44:
12 16
98.41098%
0.995112%
Mass 45:
13 16
C O16O
C O16O
12 16 17
C O O
1.17618%
98.516836%
12 18
C O16O
C O18O
13 16 17
C O O
13 17 16
C O O
0.40771%
0.080177%
12 18
C O17O
C O18O
13 17 17
C O O
13 16 18
C O O
13 18 16
C O O
0.00471%
0.407293%
Mass 48:
12 18
C O18O
13 18 17
C O O
13 17 18
C O O
0.00042%
0.000161%
Mass 49:
13 18
0.00042%
0.000396%
12 17
Mass 46:
12 16
Mass 47:
12 17
C O18O
Table B. Isotope varieties (isotopomers) of N2 in an unenriched, natural abundance
reference material (left) and in two heavy isotope-enriched samples, one enriched to 50%
15
N and the other enriched to 99% 15N.
Reference
14
15
N
N
50% Enriched
99.6337%
0.3663%
50%
50%
99% Enriched
1%
99%
Mass 28:
14
N14N
99.9926741%
25%
0.01%
Mass 29:
15
N14N
N15N
0.73258%
50%
1.98%
N15N
0.00001%
25%
98.01%
14
Mass 30:
15