Bio 10: The Fundamentals of Biology Fall 2005 - 1082
advertisement

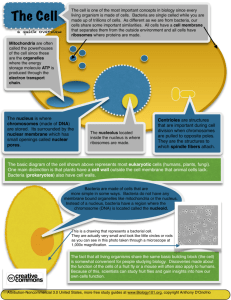
Bio 10: The Fundamentals of Biology Fall 2005 - 1082 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Fundamentals of Biology 1. Course Summary 2. Course Curriculum Inquiry into Life, th 11 edition by Sylvia Mader Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 1: The Study of Life The Characteristics of Life Life is diverse yet all living things share common characteristics: 1. 2. 3. 4. 5. 6. Living things are organized. Living things obtain *REQUIRE* energy Living things can reproduce themselves Living things respond to stimuli Living things are “homeostatic” Living things can - and do - adapt 1. Living things are organized. Q. Based on this, what things are “alive”? 2. Living things acquire materials and energy. - Photosynthetic organisms use carbon dioxide, water, and solar energy to make food. - Animals obtain nutrients and energy from food eaten. - Energy is the capacity to do work. - Many living things can convert energy to motion (or thought!). Q. Based on this, what things are “alive”? 3. Living things reproduce. - Genes (DNA) contain information needed for heredity and metabolism (those are, ALL energy-related processes). - Metabolism is all the chemical reactions in the cell. - Reproduction may be asexual or sexual. Q. Based on this, what things are “alive”? 4. Living things respond to stimuli. - Living things may respond to external stimuli by movement toward or away from a stimulus. - Movement constitutes part of the behavior of an organism. 5. Living things are homeostatic. - Homeostasis is the ability of an organism to maintain relatively constant internal conditions. (An example is temperature regulation in the human body ….or….ever feel thirsty?). - All organ systems contribute to homeostasis. 6. Living things grow AND develop. All organisms undergo development. 7. Living things are adapted. - Adaptations come about through evolution. - Evolution is the process by which a species changes through time. - Evolution explains both the unity and diversity of life. The Classification of Living Things There are three main domains: 1 and 2. Archaea and Bacteria – unicellular prokaryotes that lack a membrane-bound nucleus. 3. Eukarya – showing cellular complexity and having a nucleus and other organelles. The three domains of life Three-Domain System • The three-domain system recognizes three domains: Bacteria, Archaea, and Eukarya. • This system of classification is based on biochemical differences that show there are three vastly different groups of organisms. Archaea live in harsh environments and may represent the first cells to have evolved. Bacteria, some of which cause human diseases, are present in almost all habitats on earth. Many bacteria are important environmentally and commercially. The Domain Eukarya is divided into 4 kingdoms: Protists (kingdom Protista) Fungi (kingdom Fungi) Plants (kingdom Plantae) Animals (kingdom Animalia) The Classification of Living Things Domains (3: Archaea, Bacteria, Eukarya) Kingdom Phylum Class Order Family Genus Species The Classification of Living Things • Taxomony is the science of identifying and classifying organisms according to specific criteria using these categories: Kingdom Phylum Class Order Family Genus Species Five-Kingdom System • The five-kingdom system of classification is based on structural differences and also on modes of nutrition among the eukaryotes. • The five kingdoms include: • Monera (prokaryotes aka: bacteria) • Eukaryotic kingdoms of Protista, Fungi, Plantae, and Animalia (aka: plants and animals). The Classification of Living Things • Taxomony is the science of identifying and classifying organisms according to specific criteria using these categories: Kingdom Phylum Class Order Family Genus Species The Classification of Living Things Domains (3: Archaea, Bacteria, Eukarya) Kingdom (5) Phylum Class Order Family Genus Species Three-domain system of classification Five-kingdom system of classification The Classification of Living Things Domains Kingdom Phylum Class Order Family Genus Species Scientific names are binomial names, using genus and species. Modern humans are Homo sapiens. Most genera contain a number of similar species, with the exception of Homo that only contains modern humans. Classification is based on evolutionary relationships. Each successive classification category contains more different types of organisms than the preceding category. The Organization of the Biosphere The biosphere is the zone of life in the air, water, and land that surrounds the planet. Groups of individuals of a species are called populations. Populations of different species that interact make up communities. Communities plus the physical habitat form ecosystems. Ecosystems are characterized by chemical cycling and energy flow. Climate determines what ecosystem can exist in an area. Human populations tend to modify ecosystems for their uses. Loss of ecosystems results in loss of biodiversity, the total number of species. Preservation of ecosystems is important to ensure our continued existence. Loss of species threatens ecosystems. The Process of Science Biology, the study of life, uses the scientific method. The scientific method has these steps: Observation Hypothesis Experiments/Further Observations Conclusion Theory An experimental design contains a control group that goes through all the steps of the experiment but is not exposed to the factor being tested. Results of an experiment are called data. Data undergo statistical evaluation. Several theories in biology include: Cell Biogenesis Evolution Gene Scientific studies may be carried out in the field or in the lab. In either type of study, scientists formulate testable hypotheses, make observations or perform experiments, and come to objective conclusions. Field Study Example Controlled Laboratory Experiment Example Science and Social Responsibility Technology is the application of knowledge for a practical purpose. Technology has both benefits and drawbacks. Ethical and moral issues surrounding the use of technology must be decided by everyone. Chapter 2: The Molecules of Cells Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Basic Chemistry Matter is anything that takes up space and has weight. All matter, living or nonliving, is made up of elements. Elements contain atoms. An atom is the smallest unit of matter that can enter chemical reactions. Atoms have a central nucleus made up of protons and neutrons, and shells around the nucleus in which electrons orbit. • inner energy shell holds two electrons • outer energy shell holds eight electrons The number of electrons in the outer energy shell determines the chemical properties of the atom. Model of an Atom The atomic number of an atom is its number of protons. • protons bear a positive electrical charge The atomic weight of an atom is its number of protons plus its number of neutrons. • neutrons bear no electrical charge • electrons bear a negative electrical charge An electrically neutral atom means: number of protons = number of electrons Elements are arranged in a periodic table: • horizontally in order of increasing atomic number • vertically according to the number of electrons in the outer shell Atoms have an atomic symbol, atomic number, and atomic mass. Some atoms differ in their number of neutrons and are called isotopes. Carbon has 3 isotopes: • Carbon 12 (most abundant) • Carbon 13 • Carbon 14 (radioactive - unstable) • Atoms form bonds to fill the outer shell with electrons. • When atoms bond with other atoms, molecules are formed. • When atoms of different elements bond, a compound is formed. • Two types of bonds are ionic bonds and covalent bonds. In ionic bonding, atoms give up or accept electrons, resulting in ions. Ions with opposite charges (- or +) are attracted to each other and form an ionic bond. Ionic Bonds Ions can have important biological functions. Covalent Bonds In covalent reactions, atoms share electrons, resulting in covalent bonds. There are other ways of representing covalent bonds. Aside from single covalent bonds, double, or triple covalent bonds can form. The three-dimensional shape of molecules can be represented in two ways: Water and Living Things • Water is the most abundant molecule in living things. • Water has special traits that make it important to life. • Because oxygen atoms are large and hydrogen atoms are small, water is a polar molecule. • Hydrogen bonds form when a covalently-bonded H+ is attracted to a negatively-charged atom in a neighboring molecule. • Because of its polarity and hydrogen bonding, water has unique characteristics that benefit living things. Characteristics of water: 1. 2. 3. 4. 5. 6. liquid at room temperature universal solvent for polar molecules water molecules are cohesive (sticky) temperature of water changes slowly high heat of vaporization frozen water is less dense so ice floats Another thing water can do……………………. • Water dissociates and releases hydrogen ions (H+) and hydroxide ions (OH-). This means acids and bases can form! *Which will change the pH of solutions* • Acids are molecules that release hydrogen ions in solution. HCl H+ + Cl- • Bases are molecules that either take up hydrogen ions or give off hydroxide ions in solution. NaOH Na+ + OH- • Concentrations of hydrogen ions or hydroxide ions can be represented using the pH scale. moles/liter 1 x 10 –6 [H+] = pH 6 1 x 10 –7 [H+] = pH 7 1 x 10 –8 [H+] = pH 8 Now, back to the whole “homeostasis” thing………. • Buffers are substances that help to resist change in pH. Basic Acidic This buffer is found in YOUR BLOOD! It is used to keep your blood pH at 7.0….. If it gets to acidic, base forms….too basic, acids form. A built in mechanism for blood homeostasis! Organic Molecules Organic molecules are found in living things. The chemistry of carbon accounts for the chemistry of organic molecules. Organic molecules are macromolecules. Hydrocarbon chains can have functional groups that cause the macromolecule to behave in a certain way. (insert text art from top right column of page 31) Macromolecules (polymers) are formed from smaller building blocks called monomers. Polymer carbohydrate protein nucleic acid Monomer monosaccharides amino acid nucleotide Carbohydrates Carbohydrates serve as quick energy and short-term energy storage. They play a structural role in plants, bacteria, and insects. Monomers of carbohydrates are the monosaccharides: glucose fructose galactose Structure of Glucose Glucose A disaccharide is made from linking two monosaccharides together. Larger polysaccharides are made from linking many glucose molecules together through condensation synthesis. Examples of polysaccharides: Starch glycogen cellulose Lipids Lipids serve as long-term energy stores in cells, form membranes, and serve as hormones and insulation. Lipids do not dissolve in water. Fats and oils are formed from a glycerol molecule and three fatty acid molecules. Structure of Triglycerides Fatty acids are long chains of hydrocarbons ending in - COOH Fatty acids may be saturated fatty acids or unsaturated fatty acids. Some lipids are phospholipids that form cell membranes. Other lipids are steroids. Examples include cholesterol, and the sex hormones estrogen and testosterone. Proteins Proteins perform many functions in cells. Proteins: Serve as structural proteins Act as enzymes to speed reactions Serve as transport carriers Act as antibodies Allow materials to cross cell membranes Proteins are polymers of amino acids. Peptide bonds join amino acids. Proteins have levels of organization. Proteins can be denatured (broken down). Nucleic Acids Nucleic acids are polymers of nucleotides. Examples include Deoxyribonucleic Acid (DNA) and Ribonucleic Acid (RNA). DNA is double-stranded, with complementary base pairing. A and T ……………….G and C Some nucleotides also perform functions in cells. Adenosine triphosphate (ATP) is the energy currency of cells. Chapter 3: Cell Structure and Function Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. The Cellular Level of Organization • Living things are constructed of cells. • Living things may be unicellular or multicellular. • Cell structure is diverse but all cells share common characteristics. • The cell theory states: 1. All organisms are composed of one or more cells. 2. Cells are the basic unit of structure and function in organisms. 3. All cells come only from other cells. • Cells are small so they can exchange materials with their surroundings. Think: - How much easier is it to get to the middle of a small cell vs. a big one? Surface Area in general: Biology depends on it! Increasing surface area, increases an organisms ability To really stretch out and absorb all it can. Trust me, Whenever it can increase surface area……it will! -Example: your small intestine….it has finger-like projections that stick out to grab more. To imagine how much better this works, think of the baggage claim area of a airport… Sizes of living things Eukaryotic Cells • Eukaryotic cells have a nucleus that controls the workings of the cell. • All cells are surrounded by a plasma membrane made of phospholipids and proteins. The Plasma Membrane • The plasma membrane regulates what enters and exits the cell. • Inside the plasma membrane, the nucleus is surrounded by cytoplasm. • Plant cells have a cell wall in addition to the plasma membrane. • Animal and plant cells have organelles (literally, mini-organs!). • Organelles compartmentalize functions within the cell. (Just like our organs do!....little brain, little stomach, even a little skeleton!) • The organelles of animal and plant cells are similar to each other except that chloroplasts (which carry out photosynthesis) are present only in plant cells. Animal cell anatomy Animal cell anatomy Plant cell anatomy Plant cell anatomy Structure of the Nucleus (the brain of the cell) • Chromatin: DNA and proteins • Nucleolus: Chromatin and ribosomal subunits • Nuclear envelope: Double membrane with pores • Nucleoplasm: semifluid medium inside the nucleus. Nucleus and nuclear envelope Ribosomes (Construction workers….for protein) • Protein synthesis occurs at tiny organelles called ribosomes. • They literally READ a set of instructions sent by the nucleus to make individual proteins….each protein is individually made! • Ribosomes can be found alone in the cytoplasm, in groups called polyribosomes, or attached to the endoplasmic reticulum. The Endomembrane System (A Fed-Ex Factory System to get things, built, processed, packaged, AND delivered!) • The endomembrane system consists of: • Endoplasmic reticulum • Golgi apparatus • Vesicles The Endoplasmic Reticulum (The processing and packaging service of the cell) • The endoplasmic reticulum (ER) is a system of membranous channels and saccules. • Rough ER is studded with ribosomes and is the site of protein synthesis and processing. • Smooth ER lacks ribosomes and is the site of synthesis of phospholipids and the packaging of proteins into vesicles, among other functions. The endoplasmic reticulum The Golgi apparatus (the pancake stack-packing and shipping dept. of the cell) • The Golgi apparatus consists of a stack of curved saccules. • The Golgi apparatus receives protein and also lipidfilled vesicles from the ER, packages, processes, and distributes them within the cell. *Think….distribution or shipping dept. It puts an address on the package and sends it out!* • This organelle may also be involved in secretion. The Golgi apparatus The Endomembrane System (A Fed-Ex Factory System to get things, built, processed, packaged, AND delivered!) • The endomembrane system consists of: • Endoplasmic reticulum (built and processed) • Golgi apparatus (processed and packaged) • Vesicles (delivered!) Lysosomes and vacuoles (the cell’s stomach) • Lysosomes are vesicles produced by the Golgi apparatus. • Lysosomes contain hydrolytic enzymes and are involved in intracellular digestion. • Vacuoles are large membranous sacs in the cell that store substances. Peroxisomes • Peroxisomes are vesicles than contain enzymes. • The enzymes in these organelles use up oxygen and produce hydrogen peroxide. • Peroxisomes are abundant in the liver where they produce bile salts and cholesterol and break down fats. Energy-Related Organelles • The two energy-related organelles of eukaryotes are: chloroplasts and mitochondria. • Both organelles house energy in the form of ATP (the energy currency for ALL life!). Chloroplasts • A chloroplast is bounded by two membranes enclosing a fluid-filled stroma that contains enzymes. • These membranes house chlorophyll. • Chlorophyll absorbs solar energy and carbohydrates are made in the stroma – sooooo this is where photosynthesis takes place! Chloroplast structure Mitochondria (!!!THE POWERHOUSE!!!!) • Mitochondria are found in both, plant AND animal cells. • Mitochondria are bounded by a double membrane surrounding fluid-filled matrix – why a DOUBLE membrane?!?! - Good question! Key Point about Mitochondria ------ The matrix contains enzymes that break down carbohydrates and the cristae house protein complexes that produce ATP !!!!!! (your LIFE energy!) Mitochondrion structure The Cytoskeleton • The eukaryotic cytoskeleton is a network of filaments and tubules that extends from the nucleus to the plasma membrane. • The cytoskeleton contains three types of elements responsible for cell shape, movement within the cell, and movement of the cell: • Actin filaments • Microtubules • Intermediate filaments • Actin filaments occur in bundles or mesh-like networks. • Actin filaments play a structural role in intestinal microvilli and also interact with motor molecules, such as myosin. Actin filaments • Microtubles are small hollow cylinders made of the globular protein tubulin. • Microtubule assembly is controlled by the microtubule organizing center, called the centrosome. • Microtubules help maintain the shape of the cell and act as tracks along which organelles can move. Microtubule structure • Intermediate filaments are ropelike assemblies of fibrous polypeptides that support the plasma membrane and nuclear envelope. Structure of intermediate filaments Centrioles • Centrioles are short cylinders with a 9 + 0 pattern of microtubule triplets. • Centrioles may be involved in microtubule formation and disassembly during cell division and in the organization of cilia and flagella. Centriole structure Cilia and flagella • Cilia (small and numerous) and flagella (large and single) have a 9 + 2 pattern of microtubules and are involved in cell movement. • Cilia and flagella move when the microtubule doublets slide past one another. • Each cilium and flagellum has a basal body at its base. Structure of a flagellum or cilium Prokaryotic Cells • Prokaryotic cells include the bacteria and archaea. • • • • • • • Bacterial cells have these constant features: Outer Boundary: Cell wall Plasma membrane Cytoplasm: Ribosomes Thylakoids (Cyanobacteria) Innumerable enzymes Nucleoid: Chromosome (DNA only) • Bacterial cells may have plasmids, small accessory rings of DNA. • Some bacteria have a capsule or a slime layer. • Most bacteria have flagella. • Some also have fimbriae that help cells attach to surfaces. • Bacteria have a great metabolic diversity. Evolution of the Eukaryotic Cell • According to the endosymbiotic hypothesis, eukaryotes arose from a symbiotic relationship between various prokaryotes. - Heterotrophic bacteria became mitochondria. - Cyanobacteria became chloroplasts. - Host cell was a large eukaryotic cell. Evolution of the eukaryotic cell Lab: The Cell Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. The three domains of life Three-domain system of classification The Classification of Living Things Domains (3: Archaea, Bacteria, Eukarya) Kingdom Phylum Class Order Family Genus Species Five-Kingdom System • The five-kingdom system of classification is based on structural differences and also on modes of nutrition among the eukaryotes. • The five kingdoms include: • Monera (prokaryotes aka: bacteria) • Eukaryotic kingdoms of Protista, Fungi, Plantae, and Animalia (aka: plants and animals). The Classification of Living Things 3 Domains: Archaea Bacteria Monera Eukarya Protista Plantae 5 Kingdoms Fungi Animalia The Classification of Living Things Archaea Bacteria Monera 1. Oscillatoria 2. Gleocapsa Eukarya Protista Plantae 1. Euglena 1. Elodea 2. Paramecium 2. Potato Animalia 1. You! Fungi 1. Yeast Animal cell anatomy Plant cell anatomy Lab 4 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diffusion and Osmosis • Diffusion is the passive movement of molecules from a higher to a lower concentration until equilibrium is reached. • Gases move through plasma membranes by diffusion. Process of diffusion Gas exchange in lungs by diffusion Osmosis • The diffusion of water across a differentially permeable membrane due to concentration differences is called osmosis. • Diffusion always occurs from higher to lower concentration. • Water enters cells due to osmotic pressure within cells. Osmosis demonstration Osmosis in cells • A solution contains a solute (solid) and a solvent (liquid). • Cells are normally isotonic to their surroundings, and the solute concentration is the same inside and out of the cell. • “Iso” means the same as, and “tonocity” refers to the strength of the solution. Osmosis in plant and animal cells • Hypotonic solutions cause cells to swell and possibly burst. • “Hypo” means less than. • Animal cells undergo lysis in hypotonic solution. • Increased turgor pressure occurs in plant cells in hypotonic solutions. • Plant cells do not burst because they have a cell wall. • Hypertonic solutions cause cells to lose water. • “Hyper” means more than; hypertonic solutions contain more solute. • Animal cells undergo crenation (shrivel) in hypertonic solutions. • Plant cells undergo plasmolysis, the shrinking of the cytoplasm.