BONDING
advertisement

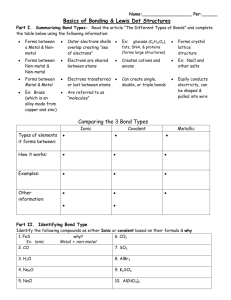
CHEMISTRY The Central Science 9th Edition Chapter 8 Basic Concepts of Chemical Bonding David P. White Prentice Hall © 2003 Chapter 8 Chemical Bonds, Lewis Symbols, and the Octet Rule • Chemical bond: attractive force holding two or more atoms together. • Covalent bond results from sharing electrons between the atoms. Usually found between nonmetals. • Ionic bond results from the transfer of electrons from a metal to a nonmetal. • Metallic bond: attractive force holding pure metals together. Prentice Hall © 2003 Chapter 8 Chemical Bonds, Lewis Symbols, and the Octet Rule • • • • Lewis Symbols As a pictorial understanding of where the electrons are in an atom, we represent the electrons as dots around the symbol for the element. The number of electrons available for bonding are indicated by unpaired dots. These symbols are called Lewis symbols. We generally place the electrons one four sides of a square around the element symbol. Prentice Hall © 2003 Chapter 8 Chemical Bonds, Lewis Symbols, and the Octet Rule Lewis Symbols Prentice Hall © 2003 Chapter 8 Chemical Bonds, Lewis Symbols, and the Octet Rule The Octet Rule • All noble gases except He has an s2p6 configuration. • Octet rule: atoms tend to gain, lose, or share electrons until they are surrounded by 8 valence electrons (4 electron pairs). • Caution: there are many exceptions to the octet rule. Prentice Hall © 2003 Chapter 8 Ionic Bonding Consider the reaction between sodium and chlorine: Na(s) + ½Cl2(g) NaCl(s) DHºf = -410.9 kJ Prentice Hall © 2003 Chapter 8 Ionic Bonding • The reaction is violently exothermic. • We infer that the NaCl is more stable than its constituent elements. Why? • Na has lost an electron to become Na+ and chlorine has gained the electron to become Cl-. Note: Na+ has an Ne electron configuration and Cl- has an Ar configuration. • That is, both Na+ and Cl- have an octet of electrons surrounding the central ion. Prentice Hall © 2003 Chapter 8 Ionic Bonding • NaCl forms a very regular structure in which each Na+ ion is surrounded by 6 Cl- ions. • Similarly, each Cl- ion is surrounded by six Na+ ions. • There is a regular arrangement of Na+ and Cl- in 3D. • Note that the ions are packed as closely as possible. • Note that it is not easy to find a molecular formula to describe the ionic lattice. Prentice Hall © 2003 Chapter 8 Ionic Bonding Prentice Hall © 2003 Chapter 8 Ionic Bonding • • • • Energetics of Ionic Bond Formation The formation of Na+(g) and Cl-(g) from Na(g) and Cl(g) is endothermic. Why is the formation of Na(s) exothermic? The reaction NaCl(s) Na+(g) + Cl-(g) is endothermic (DH = +788 kJ/mol). The formation of a crystal lattice from the ions in the gas phase is exothermic: Na+(g) + Cl-(g) NaCl(s) DH = -788 kJ/mol Prentice Hall © 2003 Chapter 8 Ionic Bonding Energetics of Ionic Bond Formation • Lattice energy: the energy required to completely separate an ionic solid into its gaseous ions. • Lattice energy depends on the charges on the ions and the sizes of the ions: Q1Q2 El k d k is a constant (8.99 x 10 9 J·m/C2), Q1 and Q2 are the charges on the ions, and d is the distance between ions. Prentice Hall © 2003 Chapter 8 Ionic Bonding Energetics of Ionic Bond Formation • Lattice energy increases as • The charges on the ions increase • The distance between the ions decreases. Prentice Hall © 2003 Chapter 8 Ionic Bonding Electron Configurations of Ions of the Representative Elements • These are derived from the electron configuration of elements with the required number of electrons added or removed from the most accessible orbital. • Electron configurations can predict stable ion formation: • • • • • Mg: [Ne]3s2 Mg+: [Ne]3s1 not stable Mg2+: [Ne] stable Cl: [Ne]3s23p5 Cl-: [Ne]3s23p6 = [Ar] stable Prentice Hall © 2003 Chapter 8 Ionic Bonding Transition Metal Ions • Lattice energies compensate for the loss of up to three electrons. • In general, electrons are removed from orbitals in order of decreasing n (i.e. electrons are removed from 4s before the 3d). Polyatomic Ions • Polyatomic ions are formed when there is an overall charge on a compound containing covalent bonds. E.g. SO42-, NO3-. Prentice Hall © 2003 Chapter 8 Covalent Bonding • When two similar atoms bond, none of them wants to lose or gain an electron to form an octet. • When similar atoms bond, they share pairs of electrons to each obtain an octet. • Each pair of shared electrons constitutes one chemical bond. • Example: H + H H2 has electrons on a line connecting the two H nuclei. Prentice Hall © 2003 Chapter 8 Covalent Bonding Prentice Hall © 2003 Chapter 8 Covalent Bonding Lewis Structures • Covalent bonds can be represented by the Lewis symbols of the elements: Cl + Cl Cl Cl • In Lewis structures, each pair of electrons in a bond is represented by a single line: H H O H N H Cl Cl H F H C H H H H Prentice Hall © 2003 Chapter 8 Covalent Bonding Multiple Bonds • It is possible for more than one pair of electrons to be shared between two atoms (multiple bonds): • One shared pair of electrons = single bond (e.g. H2); • Two shared pairs of electrons = double bond (e.g. O2); • Three shared pairs of electrons = triple bond (e.g. N2). H H O O N N • Generally, bond distances decrease as we move from single through double to triple bonds. Prentice Hall © 2003 Chapter 8 Bond Polarity and Electronegativity • In a covalent bond, electrons are shared. • Sharing of electrons to form a covalent bond does not imply equal sharing of those electrons. • There are some covalent bonds in which the electrons are located closer to one atom than the other. • Unequal sharing of electrons results in polar bonds. Prentice Hall © 2003 Chapter 8 Bond Polarity and Electronegativity Electronegativity • Electronegativity: The ability of one atoms in a molecule to attract electrons to itself. • Pauling set electronegativities on a scale from 0.7 (Cs) to 4.0 (F). • Electronegativity increases • across a period and • down a group. Prentice Hall © 2003 Chapter 8 Bond Polarity and Electronegativity Electronegativity Bond Polarity and Electronegativity Electronegativity and Bond Polarity • Difference in electronegativity is a gauge of bond polarity: • electronegativity differences around 0 result in non-polar covalent bonds (equal or almost equal sharing of electrons); • electronegativity differences around 2 result in polar covalent bonds (unequal sharing of electrons); • electronegativity differences around 3 result in ionic bonds (transfer of electrons). Prentice Hall © 2003 Chapter 8 Bond Polarity and Electronegativity Electronegativity and Bond Polarity • There is no sharp distinction between bonding types. • The positive end (or pole) in a polar bond is represented + and the negative pole -. Prentice Hall © 2003 Chapter 8 Bond Polarity and Electronegativity Dipole Moments • Consider HF: • The difference in electronegativity leads to a polar bond. • There is more electron density on F than on H. • Since there are two different “ends” of the molecule, we call HF a dipole. • Dipole moment, m, is the magnitude of the dipole: m Qr where Q is the magnitude of the charges. • Dipole moments are measured in debyes, D. Prentice Hall © 2003 Chapter 8 Bond Polarity and Electronegativity • • • • Bond Types and Nomenclature In general, the least electronegative element is named first. The name of the more electronegative element ends in –ide. Ionic compounds are named according to their ions, including the charge on the cation if it is variable. Molecular compounds are named with prefixes. Prentice Hall © 2003 Chapter 8 Prentice Hall © 2003 Chapter 8 Bond Polarity and Electronegativity Bond Types and Nomenclature Ionic Molecular MgH2 Magnesium hydride H2S Hydrogen sulfide FeF2 Iron(II) fluoride Oxygen difluoride Mn2O3 Manganese(III) oxide Prentice Hall © 2003 OF2 Cl2O3 Dichlorine trioxide Chapter 8 Drawing Lewis Structures 1. Add the valence electrons. 2. Write symbols for the atoms and show which atoms are connected to which. 3. Complete the octet for the central atom the complete the octets of the other atoms. 4. Place leftover electrons on the central atom. 5. If there are not enough electrons to give the central atom an octet, try multiple bonds. Prentice Hall © 2003 Chapter 8 Drawing Lewis Structures Formal Charge • It is possible to draw more than one Lewis structure with the octet rule obeyed for all the atoms. • To determine which structure is most reasonable, we use formal charge. • Formal charge is the charge on an atom that it would have if all the atoms had the same electronegativity. Prentice Hall © 2003 Chapter 8 Drawing Lewis Structures Formal Charge • To calculate formal charge: • • All nonbonding electrons are assigned to the atom on which they are found. Half the bonding electrons are assigned to each atom in a bond. • Formal charge is: valence electrons - number of bonds - lone pair electrons Prentice Hall © 2003 Chapter 8 Drawing Lewis Structures Formal Charge • Consider: C N • For C: • • • There are 4 valence electrons (from periodic table). In the Lewis structure there are 2 nonbonding electrons and 3 from the triple bond. There are 5 electrons from the Lewis structure. Formal charge: 4 - 5 = -1. Prentice Hall © 2003 Chapter 8 Drawing Lewis Structures Formal Charge • Consider: C N • For N: • • • There are 5 valence electrons. In the Lewis structure there are 2 nonbonding electrons and 3 from the triple bond. There are 5 electrons from the Lewis structure. Formal charge = 5 - 5 = 0. • We write: C N Prentice Hall © 2003 Chapter 8 Drawing Lewis Structures Formal Charge • The most stable structure has: • • the lowest formal charge on each atom, the most negative formal charge on the most electronegative atoms. Resonance Structures • Some molecules are not well described by Lewis Structures. • Typically, structures with multiple bonds can have similar structures with the multiple bonds between different pairs of atoms Prentice Hall © 2003 Chapter 8 Drawing Lewis Structures Resonance Structures • Example: experimentally, ozone has two identical bonds whereas the Lewis Structure requires one single (longer) and one double bond (shorter). O O Prentice Hall © 2003 Chapter 8 O Drawing Lewis Structures Resonance Structures Prentice Hall © 2003 Chapter 8 Drawing Lewis Structures Resonance Structures • Resonance structures are attempts to represent a real structure that is a mix between several extreme possibilities. Prentice Hall © 2003 Chapter 8 Drawing Lewis Structures Resonance Structures • Example: in ozone the extreme possibilities have one double and one single bond. The resonance structure has two identical bonds of intermediate character. O O O O O O • Common examples: O3, NO3-, SO42-, NO2, and benzene. Prentice Hall © 2003 Chapter 8 Drawing Lewis Structures Resonance in Benzene • Benzene consists of 6 carbon atoms in a hexagon. Each C atom is attached to two other C atoms and one hydrogen atom. • There are alternating double and single bonds between the C atoms. • Experimentally, the C-C bonds in benzene are all the same length. • Experimentally, benzene is planar. Prentice Hall © 2003 Chapter 8 Drawing Lewis Structures Resonance in Benzene • We write resonance structures for benzene in which there are single bonds between each pair of C atoms and the 6 additional electrons are delocalized over the entire ring: or • Benzene belongs to a category of organic molecules called aromatic compounds (due to their odor). Prentice Hall © 2003 Chapter 8 Exceptions to the Octet Rule • There are three classes of exceptions to the octet rule: • • • Molecules with an odd number of electrons; Molecules in which one atom has less than an octet; Molecules in which one atom has more than an octet. Odd Number of Electrons • Few examples. Generally molecules such as ClO2, NO, and NO2 have an odd number of electrons. N O N O Prentice Hall © 2003 Chapter 8 Exceptions to the Octet Rule Less than an Octet • Relatively rare. • Molecules with less than an octet are typical for compounds of Groups 1A, 2A, and 3A. • Most typical example is BF3. • Formal charges indicate that the Lewis structure with an incomplete octet is more important than the ones with double bonds. Prentice Hall © 2003 Chapter 8 Exceptions to the Octet Rule More than an Octet • This is the largest class of exceptions. • Atoms from the 3rd period onwards can accommodate more than an octet. • Beyond the third period, the d-orbitals are low enough in energy to participate in bonding and accept the extra electron density. Prentice Hall © 2003 Chapter 8 Strengths of Covalent Bonds • The energy required to break a covalent bond is called the bond dissociation enthalpy, D. That is, for the Cl2 molecule, D(Cl-Cl) is given by DH for the reaction: Cl2(g) 2Cl(g). • When more than one bond is broken: CH4(g) C(g) + 4H(g) DH = 1660 kJ • the bond enthalpy is a fraction of DH for the atomization reaction: D(C-H) = ¼DH = ¼(1660 kJ) = 415 kJ. • Bond enthalpies can either be positive or negative. Prentice Hall © 2003 Chapter 8 Strengths of Covalent Bonds Bond Enthalpies and the Enthalpies of Reactions • We can use bond enthalpies to calculate the enthalpy for a chemical reaction. • We recognize that in any chemical reaction bonds need to be broken and then new bonds get formed. • The enthalpy of the reaction is given by the sum of bond enthalpies for bonds broken less the sum of bond enthalpies for bonds formed. Prentice Hall © 2003 Chapter 8 Strengths of Covalent Bonds Bond Enthalpies and the Enthalpies of Reactions • Mathematically, if DHrxn is the enthalpy for a reaction, then DH rxn Dbonds broken - Dbonds formed • We illustrate the concept with the reaction between methane, CH4, and chlorine: CH4(g) + Cl2(g) CH3Cl(g) + HCl(g) DHrxn = ? Prentice Hall © 2003 Chapter 8 Strengths of Covalent Bonds Strengths of Covalent Bonds Bond Enthalpies and the Enthalpies of Reactions • In this reaction one C-H bond and one Cl-Cl bond gets broken while one C-Cl bond and one H-Cl bond gets formed. DH rxn DC - H DCl - Cl - DC - Cl DH - Cl -104 kJ • The overall reaction is exothermic which means than the bonds formed are stronger than the bonds broken. • The above result is consistent with Hess’s law. Prentice Hall © 2003 Chapter 8 Strengths of Covalent Bonds Bond Enthalpy and Bond Length • We know that multiple bonds are shorter than single bonds. • We can show that multiple bonds are stronger than single bonds. • As the number of bonds between atoms increases, the atoms are held closer and more tightly together. Prentice Hall © 2003 Chapter 8 Strengths of Covalent Bonds End of Chapter 8: Basic Concepts of Chemical Bonding Prentice Hall © 2003 Chapter 8