Supporting Information Intracellular Delivery of Molecules using Microfabricated Nanoneedle Arrays
advertisement

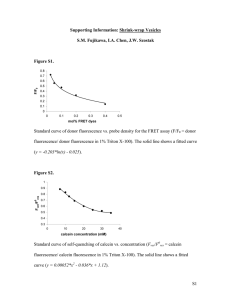
Supporting Information Intracellular Delivery of Molecules using Microfabricated Nanoneedle Arrays Seonhee Park, Seong-O Choi, Seung-joon Paik, Seungkeun Choi, Mark Allen and Mark Prausnitz S1. Materials and Methods S1.1. Analysis and quantification of puncture loading Cells were imaged using an inverted fluorescence microscope (Olympus IX70, Olympus, Center Valley, PA). Using cellSense Standard software (Olympus), images were captured using three filters – brightfield, green fluorescence, and red fluorescence – to generate images for the analysis of cell detachment, uptake of fluorescent molecules, and cell viability, respectively. The images captured were patched together to form one composite image for each of the three filters to show the entire area treated by nanoneedles. The images were then analyzed by Adobe Photoshop CS3 (Adobe Systems, Mountain View, CA) to quantify uptake and cell viability based on the cells with green and red fluorescence, respectively. The fluorescence threshold for identifying a cell as containing a dye was set based on the background fluorescence of untreated areas in the images, which served as the sham control. The percentage of cells with calcein or FITC-dextran uptake was determined by dividing the area with green fluorescence above threshold by the total area contacted by the nanoneedle array. The percentage of non-viable cells was similarly determined by dividing the area with red fluorescence above threshold by the total area contacted by the nanoneedle array and multiplying by the ratio of the nucleus area to the cell area (i.e., 0.45 in DU145 cells, as determined by microscopy), because PI only stains the nucleus of the cell. Confocal microscopy imaging was also conducted to visualize the interaction between nanoneedles and cells at higher magnification and to further study the process of intracellular uptake. The cells were plated on a glass cover slip after trypsinization and cultured for 1 - 2 days before imaging. Prior to nanoneedle puncture, the cell nucleus was stained with Hoechst 33342 (trihydrochloride, Invitrogen) at a final concentration of 2 µg/ml for 20 min and the cell membrane was subsequently stained with 1x working solution of CellMask orange or green (Invitrogen) for 10 min at 37 °C. Either propidium iodide (PI) or calcein AM (Invitrogen) was used as an uptake marker, as these dyes are fluorescent only intracellularly. Cells were either imaged live or fixed by 4% formaldehyde (Sigma-Aldrich). The cell-coated coverslip was placed on a microscope slide or a sheet of PDMS, punctured with nanoneedles and sealed with nail polish. The prepared samples were imaged using an LSM 700 confocal laser scanning microscope (Carl Zeiss Microscopy, Thornwood, NY) at 60x magnification to visualize the physical puncture by nanoneedles and associated molecular uptake. Images were captured and analyzed using the software ZEN lite black edition (Carl Zeiss Microscopy). S1.2. Analysis and quantification of centrifuge loading The uptake of fluorescent dyes and cell viability were measured using a bench-top flow cytometer (BD LSRII, BD Biosciences, San Jose, CA) based on methods described previously (Canatella et al. 2001; 1 Sengupta et al. 2014). The data were collected and analyzed in FACSDiva software (BD Biosciences). Approximately 10,000 events were collected per sample. For cell viability, PI was analyzed using a PerCP-Cy5, 670 nm longpass filter for emission. The uptake of calcein, FITC-BSA or FITC-dextran was measured by a FITC, 530/30 nm bandpass filter for emission. The cell gate was constructed based on forward- and side-scatter light of the untreated control cells. Any events within this gate were considered to be intact cells while any events outside the gate were considered to be cell debris or other noise. To determine which cells had taken up fluorescent marker compounds (i.e., calcein, BSA and dextran for uptake; PI for viability), histogram gates were set by the sham control which had fluorescent dyes in the solution but was not treated with nanoneedles to account for extracellular staining and other noise. To set gates and account for possible spectral overlap between the dyes, compensation controls were prepared and tested. The positive control was prepared by staining cells with calcein AM (Invitrogen), which stained all viable cells. The negative control was prepared by incubating cells in 70% methanol for 30 min and staining with PI. S2. Results S2.1. Cell viability after puncture loading 100 % cells 80 60 40 20 0 Control Treated Control 0 Treated 24 Time (h) Figure S1. The percentage of cells present (black) and viable cells (white) immediately after (0 h) and 24 h after treatment with and without puncture loading. The percentage is calculated relative to the cell density before treatment. Control cells were sham treated. Treated cells were punctured with nanoneedles at an applied force of 0.1 N for 2 min. Data show average ± SD, n ≥ 4. 2 S2.2. Puncture loading HeLa cells Figure S2. Representative fluorescence microscopy imaging of HeLa cells after puncture with nanoneedles. Composite (a), brightfield (b), green fluorescence (c), and red fluorescence (d) images of HeLa cells are shown after puncture loading using nanoneedles applied with a force of 0.1 N for 2 min. Green fluorescence indicates the intracellular uptake of calcein. Red fluorescence indicates PI staining, which is a marker for non-viable cells. Scale bars are 1 mm. 3 S2.3. Imaging of cells during puncture loading Figure S3. Composite (i) and green fluorescence (ii) microscopy images of DU145 cells when calcein solution was removed or added at various time points during the puncture loading experiment. (a) Calcein was replaced with fresh cell media after 1 min of puncture while nanoneedles were still in place. (b) Calcein was added 1 s after the removal of nanoneedles from the cells. (c) Calcein was added 1 min after the removal of nanoneedles from the cells. Nanoneedles were applied to the cell at a force of 0.1 N for 2 min. Dotted lines indicate the area treated with nanoneedles. Green fluorescence indicates the intracellular uptake of calcein. Scale bars are 50 µm. 4 S2.4. Transfection of cells by puncture loading Figure S4. Green fluorescence images showing the transfection of DU145 cells with plasmid DNA encoding for green fluorescent protein 24 h post-puncture with an applied force of 0.1 N for 2 min. Images (a) and (b) are representative images collected from samples that received the same nanoneedle puncture treatment. Scale bars (white) are 50 µm S2.5. Effect of nanoneedle puncture time on intracellular uptake 100 Percentage of cells 80 60 40 20 0 1 30 120 Time (s) Figure S5. Effect of nanoneedle puncture time on intracellular uptake of 70 kDa FITC-dextran and cell viability. Puncture force was 0.5 N and the down and up speeds were 150 and 10 mm/min, respectively. Data were generated from image analysis of fluorescence micrographs of cells after nanoneedle treatment. White bars indicate viable cells and black bars indicate viable cell with intracellular uptake. Data show average ± SD, n = 3. 5 S2.6. Effect of centrifugation time on intracellular uptake and viability We assessed the effect of centrifugation time on delivery efficiency and viability. Using a low centrifugal force of 65 g-force (1000 rpm), the intracellular delivery efficiency of calcein and cell viability were quantified after centrifugation times of 5 min and 20 min for treatment with nanoneedles and nanoneedle tips (Figure S6). The viability of cells collected after treatment was above 90% and did not differ significantly from the control (ANOVA, p = 0.24). We expected the longer centrifugation time to give a greater chance for cells to come in contact with nanoneedles and thereby increase calcein uptake. However, for both nanoneedles and nanoneedle tips, the delivery efficiency of calcein was not significantly different at 5 min and 20 min (Student’s t-test, p > 0.1). At both centrifuge conditions, the nanoneedles resulted in higher calcein uptake of approximately 40% compared to about 20% calcein uptake with the nanoneedle tips (Student’s t-test, p = 0.02), which is consistent with the previous data at varied centrifugal force that also showed the superiority of nanoneedles. Percentage of cells 100 80 60 40 20 0 Calcein control 5 min 20 min Nanoneedles 5 min 20 min Nanoneedle tips Figure S6. Effect of centrifugation time on intracellular uptake of calcein and cell viability. Centrifugation force was fixed at 65 g-force for centrifugation times of 5 min and 20 min. Cells were treated with nanoneedles or nanoneedle tips. Data were generated by flow cytometric analysis. White bars indicate viable cells and black bars indicate viable cell with intracellular uptake. Data show average ± SD, n ≥ 3. 6 S2.7. Intracellular uptake of FITC-BSA and cell viability Percentage of cells 100 80 60 40 20 0 FITC-BSA control Nanoneedles Nanoneedle tips Figure S7. Intracellular uptake of FITC-BSA and cell viability. Cells were treated with centrifugation at 65 g-force for 2 min using nanoneedles and nanoneedle tips. White bars indicate viable cells and black bars indicate viable cell with intracellular uptake. Data show average ± SD, n = 5. S2.8. Effect of number of cells per sample and number of treatments on intracellular uptake and viability Because calcein uptake did not exceed 40 – 50% of cells at optimal centrifugation conditions, we hypothesized that calcein uptake could be increased further by reducing the number of cells per sample and by increasing the number of centrifugation treatments in order to increase the chances of a cell encountering, and thereby being punctured by, a nanoneedle. First, we reduced the number of cells per sample by reducing the cell concentration, guided by the estimate that spinning down approximately 125,000 cells of 15 – 20 µm diameter would form a monolayer on a 5 mm x 5 mm nanoneedle array. We therefore added 62,500, 125,000, and 250,000 cells per sample in order to form approximately half, one, and two monolayers, respectively. We found that the number of cells per sample did not have a significant effect on viability compared to the untreated control (ANOVA, p = 0.12, Figure S7). Although we expected the percentage of cells with calcein uptake to increase with smaller sample size due to the increased probability of a cell becoming impaled by a nanoneedle, the delivery efficiency of calcein was also not significantly affected by number of cells per sample (ANOVA, p = 0.14), remaining approximately 40% in all samples. We next performed an experiment to test whether increasing the number of centrifugation treatments would result in higher delivery efficiency of calcein. Our hypothesis was that by increasing the number of treatments (with mixing of the cell suspension between each treatment), there would be more opportunities for a cell to come in contact with a nanoneedle for impalement. The cells were treated at 65 g-force for 2 min with both nanoneedles and nanoneedle tips using either a single centrifugation treatment or four centrifugation treatments. 7 Contrary to our hypothesis, multiple centrifugation treatments with nanoneedles and nanoneedle tips did not yield significantly different delivery efficiency of calcein compared to the single treatment (Student’s t-test, p > 0.7, Figure S7). Nanoneedle tips achieved a delivery efficiency of 20 – 30% and nanoneedles performed slightly better, with a delivery efficiency of approximately 40%. Although we were concerned that multiple treatments with nanoneedles might result in lower cell viability, viability levels of the treated samples were not significantly different (ANOVA, p = 0.074). The number of treatments and cell numbers did not have any significant change on the intracellular uptake and cell viability, possibly due to ability of the cells to deform and to adhere to the surface which would prevent the direct contact of cells with the tip of the nanoneedles and lack of mixing in the sample. This suggests that the centrifugal force is important in initially bringing cells close to the nanoneedles for puncture and is the crucial parameter in centrifuge loading method. a b 100 Percentage of cells Percentage of cells 100 80 60 40 80 60 40 20 20 0 0 Calcein control Calcein 62500 125000 250000 control Cell concentration (cells/sample) Single Multiple Nanoneedles Single Multiple Nanoneedle tips Figure S8. Effect of (a) number of cells per sample and (b) number of centrifugation treatments on the intracellular delivery of calcein and viability of DU145 cells. (a) Cell concentration was varied to achieve different numbers of cells per sample guided by their ability to form a monolayer on the nanoneedle array after being spun down. (b) Cells were spun down either one time (single) or four times (multiple). In (a) and (b), cells were treated with nanoneedles at 65 g-force for 2 min. Data were generated by flow cytometric analysis and show average ± SD, n ≥ 3. References P. J. Canatella, J. F. Karr, J. A. Petros, M. R. Prausnitz, Biophys J (2001) doi: 10.1016/S00063495(01)76055-9. A. Sengupta, S. C. Kelly, N. Dwivedi, N. Thadhani, M. R. Prausnitz, ACS Nano (2014) doi: 10.1021/Nn500100x. 8