Name ____________________ CHEM 1004 Homework #2
advertisement

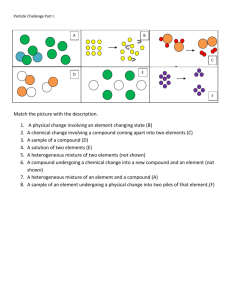
Name ____________________ CHEM 1004 Homework #2 Spring 2011 Buckley This homework is due on Wednesday, January 19, at class time. The assignment will be accepted up until the start of class on Thursday, January 20, with a 20% penalty. Assignments turned in after class that day will receive no credit, though I will look through them if you want me to. 1. (5 points) State whether each of the following properties is a physical or a chemical property. a. chlorine gas is green __________________________ b. copper wire conducts electricity _______________________ c. aluminum does not rust _________________________ d. hydrogen reacts with oxygen _____________________________ e. table salt melts at 1474 °F ___________________________ 2. (5 points) State whether each of the following undergoes a chemical change or a physical change. a. water boiling away in a pot ____________________________ b. an egg being hard-boiled c. a piece of iron rusts ____________________________ _______________________________ d. dry ice turns into vapor at room temperature _____________________ e. a nail being bent in a vice ________________________________ 3. (6 points) In the following description of zinc, indicate which of the properties indicated are physical properties and which are chemical properties. Zinc is a silver-gray-colored metal that melts at 420°C. When zinc granules are added to dilute sulfuric acid, hydrogen is given off and the metal dissolves. Zinc has a hardness on the Mohs scale of 2.5 and a density of 7.13 g/cm3 at 25 °C. It reacts slowly with oxygen gas at high temperatures to form the compound zinc oxide. 4. (10 points) For each of the following, indicate whether it is a pure substance or a mixture. For pure substances, further indicate whether the substance is an element or a compound. For mixtures, further indicate whether the mixture is homogeneous or heterogeneous. Matter a pinch of sugar dissolved in water the contents of a balloon filled with hydrogen and oxygen gas water formed when hydrogen gas is burned in the presence of oxygen gasoline a sample of Gulf of Mexico water from an area contaminated by the BP oil spill Pure Substance or Mixture? If pure substance – element or compound? If mixture – homogeneous or heterogeneous?