Agenda for Session 1
advertisement

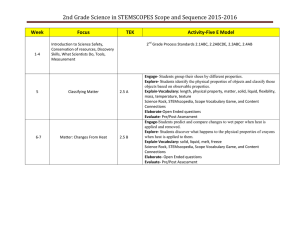
S3ETS: Supporting Science Success for Elementary Teachers and Students Today’s Agenda: January 19, 2016 Element Content Focus Content Objectives Details Standard 1: Students will understand that chemical and physical changes occur in matter. Objective 1: Describe that matter is neither created nor destroyed even though it may undergo change. Teachers will use a model to construct an explanation for how matter is conserved through a living system. Teachers will find patterns in CO2 and O2 levels that give evidence that a chemical change has occurred by analyzing and interpreting data. Teachers will use mathematical thinking and data to find patterns in the arrangement of the Periodic Table of Elements. Language Objectives Teachers will correctly define “The Law of Conservation of Mass”. Session Sequence 1. Welcome!- Louisa Stark Teachers will read the x-axis and y-axis correctly 2. Overview and Grant Requirements- Candace Penrod 3. S3ETS Business- Dina Drits 4. Engage- The Radish Problem 5. Explore-What is photosynthesis? What is cellular respiration? 6. Explain- Why are the seed masses different? 7. Extend/Elaborate- Open vs closed system (H2O2 + liver) 8. Evaluate- What new ways can teach your students about conservation of mass? What then, is a chemical property vs a physical property? a. Atomic Structure Reading Periodic Boxes b. Incomplete Radish Problem a. Seeds and Predictions b. Show masses- Formulate explanations c. 5. Video 6. Candle Demo 7. Assignment- The weight of an object is always equal to the sum of its parts, regardless of how it is assembled. In a chemical reaction or physical change matter is neither created nor destroyed. When two or more materials are combined, either a chemical reaction or physical change may occur. Investigate evidence for changes in matter that occur during a chemical reaction. http://periodic.lanl.gov/images/periodictable.pdf