atomic theory
advertisement

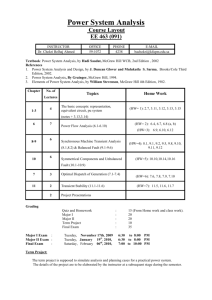
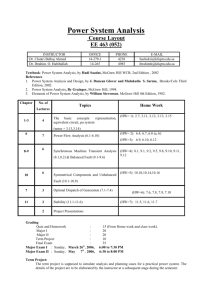
1.3 Atomic Theory • Early ideas about matter • The Greek philosopher Democritus believed that matter was made of atomos that were the smallest pieces of matter. • Atomos is a Greek word that means UNCUTTABLE! • Alchemists experimented with matter and tried to turn common metals into gold. Their activities marked the beginning of our understanding of matter. See pages 28 - 29 (c) McGraw Hill Ryerson 2007 Democritus • Democritus claimed that everything is made up of atoms. • These atoms are physically indivisible; • between atoms lies empty space; (c) McGraw Hill Ryerson 2007 Democritus • atoms are indestructible; • have always been, and always will be, in motion • there are an infinite number of atoms and kinds of atoms, which differ in shape, and size. (c) McGraw Hill Ryerson 2007 Development of Atomic Theory I • John Dalton (1766 - 1844) • Credited with developing a theory that was a new way of explaining matter. • He studied gases that make up Earth’s atmosphere. Based on his studies, he suggested that: • matter is made of small, hard spheres that are different for different elements • the smallest particle of an element is called an atom • This is the basis for Dalton’s Atomic Theory. See page 29 (c) McGraw Hill Ryerson 2007 Dalton’s Atomic Theory 1. All matter is made of small particles called atoms. 2. Atoms cannot be created, destroyed, or divided into smaller particles. 3. All atoms of the same element are identical in mass and size, but they are different in mass and size from the atoms of other elements. 4. Compounds are created when atoms of different elements link together in definite proportions. See page 30 (c) McGraw Hill Ryerson 2007 Atomic Theory II • J. J. Thomson (1856 - 1940) • Thomson studied electric currents in gas discharge tubes (like today’s fluorescent lights). From his studies, he determined that the currents were streams of negatively charged particles. These were later called electrons. • He hypothesized that atoms are made of smaller particles. He proposed the “raisin bun” model of the atom. • This model is best visualized as a positively charged bun with negatively charge particles spread out in it like raisins. See page 30 (c) McGraw Hill Ryerson 2007 Atomic Theory III • Ernest Rutherford (1871 - 1937) • After experimenting with charged particles, he found that some particles were deflected in directions not originally predicted. • He suggested that the deflection of the charged particles was because the atom contained a tiny dense centre called a nucleus, and electrons moved around the nucleus. See page 31 (c) McGraw Hill Ryerson 2007 Atomic Theory IV • Niels Bohr (1885 - 1962) • He studied gaseous samples of atoms, which were made to glow by passing an electric current through them. • Based on his observations, Bohr proposed that electrons surround the nucleus in specific “energy levels” or “shells.” See page 31 - 32 (c) McGraw Hill Ryerson 2007 The Planetary Model • The Bohr Model is a planetary model in which the negatively-charged electrons orbit a small, positively-charged nucleus similar to the planets orbiting the Sun (except that the orbits are not planar). (c) McGraw Hill Ryerson 2007 The Planetary Model • The Bohr model shows that the electrons in atoms are in orbits of differing energy around the nucleus (think of planets orbiting around the sun). • Bohr used the term energy levels (or shells) to describe these orbits of differing energy. He said that the energy of an electron is quantized, meaning electrons can have one energy level or another but nothing in between. (c) McGraw Hill Ryerson 2007 The Planetary Model • Bohr suggested that electrons will occupy these energy levels (shells) when surrounding the nucleus. • However, each shell that surrounds the nucleus can ONLY hold a specific number of electrons. • This turns out to be a very big deal for us because it ultimately determines why and how compounds are made. (c) McGraw Hill Ryerson 2007 Specific # of Electron per shell • Due to shell characteristics, Bohr determined each shell around the nucleus can hold the following: • Shell 1 = 2 Electrons • Shell 2 = 8 Electrons • Shell 3 = 8 Electrons • Shell 4 = 18 Electrons • Shell 5 = 18 Electrons (c) McGraw Hill Ryerson 2007 The Planetary Model (c) McGraw Hill Ryerson 2007 The Planetary Model • The energy level an electron normally occupies is called its ground state. But it can move to a higher-energy, less-stable level, or shell, by absorbing energy. This higher-energy, lessstable state is called the electron’s excited state. • After it’s done being excited, the electron can return to its original ground state by releasing the energy it has absorbed, as shown in the diagram on the next slide! (c) McGraw Hill Ryerson 2007 Energy Storage and Release (c) McGraw Hill Ryerson 2007 Inside the Atom • An atom is the smallest particle of an element that retains the properties of the element. • All atoms are made up of three kinds of particles called subatomic particles. These particles are: • electrons • protons • neutrons Take the Section 1.3 Quiz (c) McGraw Hill Ryerson 2007 See pages 32 - 33