Assessment of current T-stage parameters I. Peripancreatic soft tissue (PST) involvement
advertisement

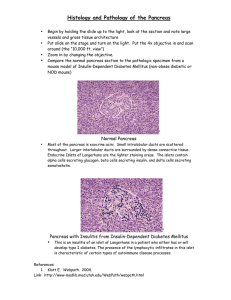
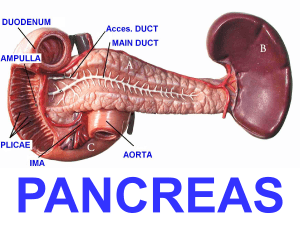
Assessment of current T-stage parameters I. Peripancreatic soft tissue (PST) involvement Definition; current guidelines The most detailed explanation of the PST appeared in the CAP webpage, where it was defined as “invasion of soft tissues adjacent to pancreas” and “include the surrounding retroperitoneal fat (retroperitoneal soft tissue)…” 1. It was presumed that, in a pancreatoduodenectomy specimen, the “PST” essentially referred to the soft tissues covering the pancreatic head. Application of “PST” involvement Both during our daily practice and during the execution of this study, it became clear that the PST is poorly defined and difficult to assess because: 1) Pancreas does not have a capsule and therefore there is no well-defined “peri”-pancreatic soft tissue layer distinguishable anatomically by gross or microscopic examination. Considering that the assessment of extra-organ extension presents substantial challenges in daily practice even in capsulated organs such as the prostate 2, and thyroid 3,4, this problem is magnified in the pancreas. 2) Pancreatic lobules are highly irregularly distributed in the pancreas. Depending on the patient (presumably age, diet and other factors), there is also often variable degree of atrophy and therefore it is impossible to determine the true outlines of the organ. 3) Adipose tissue is often abundant in between pancreatic elements, and become even more abundant in some patients 5-8. 4) This already ill-defined interface is further obscured by peritumoral inflammation and fibrosis in a significant proportion of the cases. Prior biopsy/instrumentation related changes or chronic pancreatitis that precedes the cancer formation in some patients also contribute to this challenge (Supplemental Figure 1). Therefore, depending on how the histologic sections are obtained and oriented, there are tremendous variations in what can be regarded as PST. II. Common bile duct (CBD) involvement Definition; current guidelines The evaluation of the current guidelines on the CBD parameter revealed highly conflicting information. In the AJCC staging book, there were no specifications, leading to the conclusion that any part of the CBD is to be considered pT3 9. In contrast, in the page 12 of the CAP protocol, it was specifically stated as “extrapancreatic biliary system” 1. However, in the next page of the same protocol, the illustration of pT3-CBD-involvement documents a tumor that appeared to be localized in the intrapancreatic segment of CBD 1. In the AFIP fascicle, the authors state that “The involvement of the intrapancreatic portion of the bile duct does not signify extension beyond the pancreas in the T classification of carcinomas of the pancreas. Involvement of the bile duct should be designated as T3 only when the carcinoma extends beyond the pancreas to involve the extrapancreatic biliary tree” 10. However, personal communication with the authors (David Klimstra, March, 2014) reveals that they were also not certain as to how this ought to be interpreted. Application of CBD involvement in practice It became clear during this study that that there is no defined boundaries between the “extra” and “intra-pancreatic” segment of CBD and therefore they are anatomically and histologically indistinguishable. The pancreas surrounding the CBD is highly irregular in distribution and depending on the sectioning, vastly different impressions can be obtained. Thus, involvement of “extra-pancreatic biliary system” is not a reproducible parameter. III. Involvement of duodenum/ampulla Although it is not clearly stated in many of the guideline texts and thus practice varies greatly on whether this refers to the mucosa-only or the muscularis as well, in the CAP protocol, it is stated as “Tumor invades duodenal wall”, presumed to refer to the muscularis involvement as well 1. References 1. Washington K, Berlin J, Branton P, Burgart LJ. CAP cancer reporting protocols: protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. 2013; http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebC ontent/pdf/pancreasexo-13protocol-3201.pdf. 2. Evans AJ, Henry PC, Van der Kwast TH, et al. Interobserver variability between expert urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens. Am J Surg Pathol 2008;32:1503-1512. 3. Mete O, Rotstein L, Asa SL. Controversies in thyroid pathology: thyroid capsule invasion and extrathyroidal extension. Ann Surg Oncol 2010;17:386-391. 4. Mete O, Asa SL. Pitfalls in the diagnosis of follicular epithelial proliferations of the thyroid. Adv Anat Pathol 2012;19:363-373. 5. Altinel D, Basturk O, Sarmiento JM, et al. Lipomatous pseudohypertrophy of the pancreas: a clinicopathologically distinct entity. Pancreas 2010;39:392-397. 6. Adsay NV, Basturk O, Klimstra DS, Kloppel G. Pancreatic pseudotumors: non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin Diagn Pathol 2004;21:260-267. 7. Kuroda N, Okada M, Toi M, Hiroi M, Enzan H. Lipomatous pseudohypertrophy of the pancreas: further evidence of advanced hepatic lesion as the pathogenesis. Pathol Int 2003;53:98-101. 8. Flohr T, Bonatti H, Shumaker N, et al. Liver transplantation in a patient with primary sclerosing cholangitis suffering from lipomatous pseudohypertrophy of the pancreas. Transpl Int 2008;21:89-91. 9. Edge SB, Byrd DR, Compton CG, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7 ed. New York: Springer, 2010. 10. Hruban R, Pitman MB, Klimstra DS. Tumors of the pancreas. Washington, DC: American Registry of Pathology, 2007.