Reiff_Metal Ion Insertion.ppt
advertisement

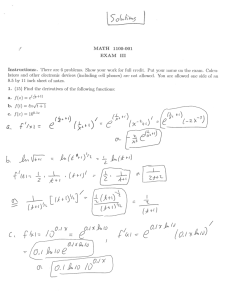
Reversible, Reductive, Topotactic Metal Ion Insertion Chemistry of Ferric Networks à La Chimie Douce aWilliam M. Reiff, aLázlo Tákács, aJános Tákács, aKάroly Lázár, and bC.C. Torardi aDepartment of Chemistry, Northeastern Uiversity , Boston, MA 02115 ,USA, w.reiff @ neu.edu, and bCentral Research and Development Department, E. I. du Pont de Nemours and Co., Experimental Station, Wilmington, DE 19898, USA Topotactic metal ion insertion is an important approach to the preparation of fast ion conductors of potential utility in high energy density devices. D.W. Murphy and P.A. Christian, Science, 205, 651(1979). M. M. Thackeray, W. I. F. David, and J. B. Goodenough, J. Solid State Chem. 55, 280 (1984). In addition to lithiation of various spinels either electrochemically or chemically e.g. via reaction with butyl-lithium, distinctly milder reagents and reaction conditions such as LiI (dissolved in nonpolar solvents such as acetonitrile), have proven useful as synthons for lithiation. This is so-called “Chimie Douce” (soft chemistry). Such reactions often proceed topotactically, i.e. they have the advantage of largely if not completely preserving the structural elements and symmetry features of the reactants in the products although the overall composition necessarily changes. We have used LiI to successfully , stoichiometrically lithiate the tetragonal 2D- layered chloro-molybdate, Fe(III)ClMoO4 and the monoclinic 3D network (Fe(III))2(MoO4)3 to the corresponding new ferrous phases, LiFe(II)ClMoO4 and Li2(Fe(II))2(MoO4)3, (A) and (B), respectively. C. C. Torardi, W. M. Reiff, K. Lazar, and E. Prince, J. Phys. Chem. Solids 47, 741(1986). W. M. Reiff, J. H. Zhang, and C. C. Torardi, J. Solid State Chem., 62, 231 (1986). Powder neutron diffraction study shows that the Li+ cations of (A) reside in six coordinate LiO4Cl2 sites between the Fe(III)ClMoO4 layers while in (B) are present in LiO4 tetrahedra formed via Li+ bridging edges of two FeO6 octahedra in the Fe2(MoO4)3 network as in the following figures. Fe(III)ClMoO4) (S, slurry, LiI (CH3CN soln) LiFe(II)ClMoO4(S) + LiI(soln) Five Coordinate Iron(III) (FeO4Cl) Six Coordinate Iron (II) (transFeO4Cl2) (Fe(III))2 (MoO4)3 (S, slurry) + 2 LI ( CH3CN soln) Six Coordinate Iron(III) Li2(Fe(II))2(MoO4)3 (S) + I2 (soln) Six Coordinate Iron(II) The utility of the Mössbauer Effect in following the course of lithium and proton insertion reactions for ferric networks is illustrated in the following figures, where aliquots of the reaction slurry are taken as a function of time for the specific case of monoclinic anhydrous Fe2(SO4)3, whose structure is very similar to that of Fe2(MoO4)3. Time * The above spectra* indicate that the high-spin Fe2+ product on the left has two iron sites while on the right there is only one. The following figures show that the Fe2+products of the insertion reactions are interesting magnetic materials in their own right. The ~ zero field ac susceptibility data on the right confirm that while the lithiation has little effect on TN of Fe2(MoO4)3, its magnetic ground state is strongly altered from anti-ferromagnetic to weakly ferromagnetic vis à vis ”. TN~12.5K ”~0 Lithiation TN~12.5K ”≠0 3D Ordering of Li2Fe2(Mo4)3. Behavior below TN suggests a perturbation scheme where EQ~ Hint. Summary It is clear that the Mössbauer Effect is an ideal tool for facile study of aspects of the metal ion insertion and intercalation chemistry of iron based networks. For instance, this vignette readily shows that the reductive heterogeneous reaction in which H+ ions are effectively inserted into the voids in the (Fe(III))2(SO4)3 structure: HI (g, rigorously dry) + Fe(III)2(MoO4)3 (s, in a slurry on a sintered glass frit) H2(Fe(II))2(Mo4)3 (s) + I2(s) goes to completion! In the context of “la chimie douce”, the Mössbauer Effect should prove invaluable in the investigation of other potential simple synthons, e.g. LiNO2 and HNO2 ,provided their solubility and redox properties vis à vis Fe(III) are favorable.