Lecture 21 Power point notes
advertisement

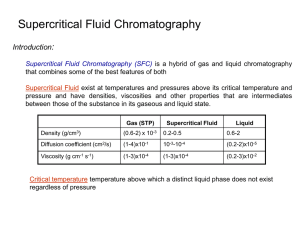
Supercritical Fluid Chromatography • Theory • Instrumentation • Properties of supercritical fluid Critical temperature Above temperature liquid cannot exist Vapor pressure at critical temperature is critical pressure T and P above critical T and P Critical point Supercritical fluid 19-1 Supercritical fluid • Above the critical temperature no phase transition regardless of the applied pressure • supercritical fluid is has physical and thermal properties that are between those of the pure liquid and gas fluid density is a strong function of the temperature and pressure diffusivity much higher a liquid readily penetrates porous and fibrous solids Low viscosity Recovery of analytes Return T and P 19-2 Typical Supercritical Solvents Compound Tcº C Pc atm d* CO2 C2H4 N2O NH3 31.3 9.9 36.5 132.5 72.9 50.5 72.5 112.5 0.96 --0.94 0.40 n-C5 n-C4 CCl2F2 196.6 152.0 111.8 33.3 37.5 40.7 0.51 0.50 1.12 CHF3 H2O 25.9 374.1 46.9 218.3 ------19-3 Supercritical fluid chromatography • Combination of gas and liquid • Permits separation of compounds that are not applicable to other methods Nonvolatile Lack functional groups for detection in liquid chromatography 19-4 Supercritical Fluid Extraction • • • • near the critical point properties change rapidly with only slight variations of pressure. inexpensive, extract the analytes faster environmentally friendly sample is placed in thimble supercritical fluid is pumped through the thimble extraction of the soluble compounds is allowed to take place as the supercritical fluid passes into a collection trap through a restricting nozzle fluid is vented in the collection trap solvent to escapes or is recompressed material left behind in the collection trap is the product of the extraction batch process 19-5 Capillary Electrophoresis • Separations based on different rate of ion migration Capillary electrochromatography separates both ions and neutral species Electroosmotic flow of buffer acts as pump • Principles • Applications 19-6 Planar electrophoresis • porous layer • 2-10 cm long paper cellulose acetate polymer gel soaked in electrolyte buffer • slow • difficult to automate 19-7 Capillary Electrophoresis • narrow (25-75 mm diameter) silica capillary tube 40-100 cm long • filled with electrolyte buffer • fast • complex but easy to automate • quantitative • small quantities nL 19-8 Separation • Movement of ions function of different parameters molecular weight charge small/highly-charged species migrate rapidly pH Deprotonation HAH+ + A ionic strength low m few counter-ions low charge shielding high m, many counter-ions high charge shielding 19-9 Migration rate • v= migration velocity me=electrophoretic mobility (cm2/Vs) • E=field strength (V/cm) • For capillary V=voltage L=length • Electrophoretic mobility depends on net charge and frictional forces Size/molecular weight of analyte Only ions separated • Plate height (H) and count (N) 19-10 Function of diffusion and V Plates • Planar electrophoresis large cross-sectional area short length low electrical resistance, high currents Sample heating Vmax=500 V N=100-1000 low resolution • Capillary electrophoresis small cross-sectional area long length • high resistance • low currents Vmax=20-100 kV • N=100,000-10,000,000 high resolution As comparison, HPLC N=1,000-20,000 19-11 Zone Broadening • Single phase (mobile phase) - no partitioning • three zone broadening phenomena longitudinal diffusion transport to/from stationary phase multipath • planar no stationary phase • capillary no stationary phase or multipath 19-12 Transport • • • • ions migrating in electric field cations to cathode (-ve) anions to anode (+ve) Electroosmosis movement in one direction anode (+ve) to cathode (-ve) Components Analyte dissolved in background electrolyte and pH buffer Silica capillary wall coated with silanol (Si-OH) and SiO Wall attracts cations double-layer forms Cations move towards cathode and sweep fluid in one direction Electroosmotic flow proportional to V usually greater than electrophoretic flow 19-13 Bulk flow properties hydrodynamic ion buffer 19-14 Techniques • Electropherogram migration time analogous to retention time in chromatography • Isoelectric focusing Gradient No net migration pH gradient with weak acid 19-15 Techniques 19-16