Answers (Word)
advertisement

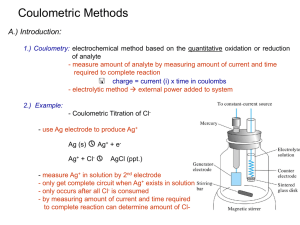
Homework 11: Chapter 23 Potentiometry, Chapter 24 Coulometry, Chapter 25 Voltammetry, Chapter 26 Chromatographic separations, Chapter 27 Gas Chromatography, Chapter 28 HPLC, Chapter 29 SCFE, Chapter 30 Capillary Electrophoresis, Chapter 31 Thermal Methods Assigned 26 April; Due 1 May 2006 1. What are suitable properties of a reference electrodes? Known half-cell so comparisons with measured potential can be made Insensitive to solution under examination. The electrode response should be reversible and obey Nernst equation. The potential should be constant under the examined condition and return to the original potential. 2. In equations for measuring ion concentration by specific electrode how is the ion concentration evaluated? The response is based on the Nernst equation so is temperature dependent and a function of the number of electrons involved in the reaction. From the Nernst equation the log of the concentration impacts the potential. From this the negative of the log of the concentration (pX) is evaluated. 3. What are some ions that can interfere with a pH measurement? Monovalent cations can interfere: Na+, K+, NH4+, Cs+, Rb+, Li+, Ag+ 4. What is a liquid membrane electrode? The electrode has a porous membrane that is used to separate liquids. The electrode response is based on the potential that develops across two immiscible liquids with different affinities for the analyte, ideally with selective bonding of the analyte. An example is calcium dialkyl phosphate which is insoluble in water, but binds Ca2+ strongly. This can be used for a Ca2+ specific electrode. 5. What is the basis of coulometry and what are 2 coulometric methods? Coulometry is the quantitative conversion of ion to new oxidation state. The amount of electrons needed provides information that can be used to determine the ion concentration. Two methods are constant potential coulometry (Potentiostatic coulometry) and constant current coulometry (Coulometric titration). 6. A Cu2+ solution is exposed to a current of 0.5 A for 500 seconds. How much Cu is deposited on the cathode? Current = 0.5 A, t= 500 s Q= 0.5(500) =250 C 2 electron reduction, n=2 and F=96485 C/mole eN = 250/(2*96485) = 1.30E-3 moles Cu2+ 7. How can the Nernst equation be used to determine the number of electrons involved in a redox reaction? The potential plotted against the log of the redox concentrations should yield a slope of -0.0592/n where n is the number of electrons. 8. How can the half-cell potential be determined from a voltammogram? The half-wave potential can be evaluated to determine the half-cell potential. On a plot of current versus applied potential the potential at half the limiting current height is the half-wave potential. The standard potential is determined by normalizing the reference used in the voltammogram to SHE. 9. In the CV below label the cathodic peak potential and the anodic peak potential. The cathodic peak potential is due to the reduction of the compound and is labeled D in the figure. The anodic peak potential is driven by the oxidation of the sample and is point B in the figure. The difference between the peak potentials in volts is 0.0592/n where n is the number of electrons involved in the half-reaction. 10. What terms are responsible for zone broadening in chromatography? Multipath term where analyte molecules can take different paths, diffusion which is a function of time on the column, and a mass transfer is related to the time it takes for the analyte to reach equilibrium on the column. 11. You have a 10 cm liquid chromatography column. You have the following data Compound tr Width of Peak Base (Wt) (min) N (minutes) A B C D 3.2 6.7 8.9 25.3 0.18 0.37 0.48 1.4 5056.79 5246.457 5500.694 5225.224 5257.292 A. Calculate the theoretical plates for each peak and the column average B. The average plate height for the column N=16(tr/Wt)2 H=L/N= 10 cm/5257 =0.0019 cm 12. What instrumentation is used in gas chromatography? A carrier gas for the analyte is needed. These gases include He, N2, and H2. A column with silica beads or functionalize surfaces is required. An oven to keep the operating temperature appropriate is needed. Finally a method to detect the analyte is required. 13. What physical properties of molecules are exploited in HPLC Size and polarity are used to achieve separations in HPLC. Solvent polarity can be varied to exploit differences in analyte polarity. The column particle size is used for size exclusion and hence separation based on molecular size 14. What is a supercritical fluid? A supercritical fluid has physical and thermal properties that are between those of the pure liquid and gas. The fluid density is a strong function of the temperature and pressure of the state for creating the SCF. The SCF has a diffusivity that is radically different from a liquid and the SCF readilypenetrates porous and fibrous solids. 15. What is needed for capillary electrophoresis? The set up includes a power supply for the potential, two electrodes, and a medium for the analytes to traverse. 16. What is the basis of thermogravimetric analysis? TGA involves monitoring the weight loss of the sample in a given atmosphere as a function of temperature. The components of the molecule can be identified by the mass loss at a given temperature or through the analysis of the off-gas.