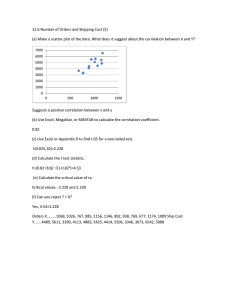
Supplementary Material for: Correlation of neuronal firing rate,
advertisement

Supplementary Material for: Correlation of neuronal firing rate, firing rate correlation, and synchrony in area MT with directional choices during stimulus and reward expectation Thiele, A., Hoffmann, K.-P. Definition of early and late SIDs (and its influence on neuronal choice probabilities) The definition of early and late SIDs in the main paper was based on the maximum of the cumulative hazard function plus 500 ms. The additional 500 ms were added because we reasoned that the resulting cut-off more appropriately reflects the internalized time of decisions in relation to the last possible stimulus presentation. This reasoning is based on decision times in relation to low (2%) and medium (4%) contrast stimulus presentation. Such decisions followed stimulus onset by ~350-500 ms 1. Although we believe that it is reasonable to assume that their internalization may be based on the percept/decision/motor response time following these low contrast stimuli, the animals might have had different mechanisms to determine and internalize the maximum of the cumulative hazard function. To control for the possibility that the cut-off used in the main paper was arbitrary and different cut-off points would generate different results in relation to firing rate differences and choice probabilities, we used a variety of different cut-off points. We calculated the choice probability associated with early and late SIDs, whereby the maximum of the cumulative hazard 1 function divided the two types of SIDs (i.e. the maximum of the hazard function itself was taken as the cut off point), or when a variety of different delays relative to the cumulative hazard function maximum divided them (Supplementary table 1). As a further control we defined early and late SIDs for each experimental session based on the animals’ overall SID timing distribution during that session. Here SIDs were subdivided into two groups of equal size, separated by the median SID time that occurred in the specific experiment. The latter approach ensured equal numbers of early and late SIDs in PD and APD direction, and also accounted for the fact that the exact speed of SIDs varied somewhat between experimental sessions. The analysis of these different cut-off times demonstrates that the finding reported in the main paper is robust with respect to the exact choice of early and late SID definitions. The ROC values in Supplementary Table 1 show that any cut-off from the maximum of the cumulative hazard function up to 600 ms thereafter yields significantly larger ROC values for early than for late SIDs. When cut-offs before the maximum of the cumulative hazard function were chosen early SIDs started to contribute to the ‘late SID’ ROCs, and the difference between the two groups decreased, cut off’s more than 600 ms after the maximum of the cumulative hazard function resulted in late SIDs contributing to the ROC calculation for early SIDs, and the differences between the ROC distributions decreased and became non-significant. Definition of SIDs (cut-off between early and late) Cumulative hazard function maximum -200 ms Cumulative hazard function maximum Cumulative ROC early SIDs ROC late SIDs (n=89) p-value for difference (signed rank test) Significantly affected cells (permutation test) n early: 11 n late: 9 0.541 +/-0.15 n=52 0.516 +/0.083 P=0.159 0.530 +/- 0.12 n=89 0.503 +/-0.089 n=89 P<0.043 n early: 15 n late: 15 0.549+/-0.122 0.503 +/- P<0.001 n early: 27 2 hazard function maximum +200 ms Cumulative hazard function maximum +400 ms Cumulative hazard function maximum +500 ms Cumulative hazard function maximum +600 ms Cumulative hazard function maximum +800 ms Split according to the median SID time for each session n=124 0.085 n=124 n late: 13 0.542+/-0.115 n=135 0.504 +/0.088 n=135 P=0.003 n early: 26 n late: 14 0.546+/-0.107 n=138 0.507 +/0.082 n=138 P<0.001 N early: 29 N late: 19 0.542+/- 0.102 n=133 0.509 +/-0.086 n=133 P=0.015 N early: 28 N late: 14 0.534+/- 0.107 n=118 0.512 +/-0.099 n=118 P=0.101 N early: 25 N late: 8 0.541 +/- 0.101 n=164 0.504 +/0.083 n=164 P<0.001 N early:32 N late: 16 Supplementary Table 1: Effect of choosing different cut-off points to define early and late SIDs on the choice probabilities. Early SIDs resulted in larger choice probability values for all the cut-offs shown in the table. However, the difference was only significant for cut-off points that started when the cumulative hazard function had maxed out until 600 ms thereafter. We argue that the decrease with very early cut-offs occurs because then early SIDs trials contaminate the late SID trials, while with later cut-offs late SID trials contaminate the early SID trials. SID timing and associated reward probabilities It could be argued that pooling SIDs across different sessions may have confounded the results in relation to the reward yield for early and late SIDs. This is because animals might have indicated more early SIDs during the early part of each experimental session (where they were more thirsty and possibly less patient, and also were likely to have forgotten the choices from the previous session), while they would indicate more late SIDs during the late part of the session, where they had better 3 memory regarding previous choices and thus reward probabilities, as well as having reduced thirst levels. To control for this possibility we subdivided each session into two equal halves, and analysed the time of choice for each of these halves in conjunction with the predicted reward magnitudes that would have been associated with each of these choices. Contrary to the proposal that thirst results in impatience, and thus more early SIDs during the first half of each session, both monkeys waited significantly longer in the first half of each session before indicating SIDs (Supplementary table 2). monkey Second half of choices (SID time) 2711+/- 732 ms (n=3253) Rank sum test CS First half of choices (SID time) 2874 +/- 661 ms (n=3241) AR 3074 +/- 668 ms (n=4632) 3026 +/- 658 ms (n=4677) P<0.001 P<0.001 Supplementary table 2: Average time of SIDs occurrence calculated separately for the first half of each experimental session and for the second half of the experimental session. Both monkeys waited significantly longer before they indicated a SID during the first half of the sessio, than during the second half of the session. This indicates that, although they may have been thirstier during the first half, this did not result in impatience, whereby they were more likely to indicate a SID early on in the hope to obtain a reward. To the contrary, it could indicate that they were more accurate, such that they only indicate early SIDs when they were sure that a stimulus had been presented, while they waited otherwise for the hazard function to max out before indicating a SID. Waiting time during the experimental session and associated reward likelihood. It might moreover be argued that the differences in SID timing which occurred during a single session (with monkeys being slightly more patient early in every session), could account for the difference in reward yield on its own, i.e. the first half of every session resulting in a different reward yield than the second half, without any direct 4 relation to early vs. late SIDs. Contrary to such a proposal we found no significant difference between the reward magnitudes that were associated with the two different groups (first half of SIDs of each daily session vs. second half of SIDs of each daily session). However, when subdividing each of these two daily halves into early vs. late SIDs (taking the cut offs described in the main paper), we found that the predicted reward magnitude was significantly larger with late SIDs for both monkeys for either half of a session (first half and second half) when compared to either of the early SID groups (1-way ANOVA, p<0.01, Tukey’s test). At the same time there was no significant difference regarding the predicted reward magnitude between the two early SID groups and there was no significant difference when comparing the predicted reward magnitude between the two late SID groups (p>0.05, post-hoc testing, Tukey’s test). We take this as evidence that our results reported in the main paper were not due to the fact that timing of SIDs changed during the course of a daily session. Rather, increases in predicted reward probability were due to the fact that a choice corresponded to a late SID and not to an early SID. Eye movement controls During the task the animals were required to fixate and keep their eyes within +/- 1° of the fixation spot. Eye movements occur within even smaller windows, and previous studies have shown that small eye-movements (within +/- 1.5° windows) across structured randomly moving backgrounds can elicit neuronal activity in area MT 2. Thus eye movements across the structured background might have elicited neuronal activity in MT on some trials, which may have triggered the animal’s response. To analyse whether eye-movements may have been responsible for our 5 results we took 2 different approaches. Firstly, we excluded trials from our sample during which eye-movements or eye position deviated by more than +/- 0.15° from the fixation spot during the last 700 ms to 100 ms prior to SIDs. We thus generated a sample of trials that was virtually free of eye movements. The procedure slightly reduced our cell sample, because we still required a minimum of 5 trials for each SID type for each cell recorded (n=122 cells instead of 138). For this selection of trials (and cells) we found the same pattern of results as described in the main paper. The median activity associated with the different SID types following additional eyemovement control is presented in table 1 of the main paper. The activity ratios between the different SID groups also produced similar results to those reported in the main paper when trials with eye-movements were eliminated. The mean of the log ratios of the PDearlySID/APDearlySID distribution was 0.207, it was 0.055 for the PDlateSID/APDlateSID, 0.104 for the PDearlySID/PDlateSID, and -0.048 for the APDearlySID / APDlateSID distribution (H0: μPDearlySID = μPDlateSID = μAPDearlySID = μAPDlateSID; p<0.001, RM ANOVA Tukey’s test). It could be argued that microsaccades or small pursuit eye movements can still occur within the remaining window of +/- 0.15°, and the related retinal motion might be sufficient to result in systematic activity changes in directionally selective MT neurons 2. As an additional control we thus eliminated trials from the sample where either (A) the eye-velocity exceeded 10°/s during the period from 700 ms to 100 ms prior to the SID thereby eliminating trials where microsaccades could confound the result, or (B) the eye position changed by more than 0.1° during any 50 ms period (equivalent to an eye drift with a velocity of >=2°/s) from 700 ms to 100 ms prior to the SID. For these eye-movement analyses we employed the same procedures as described by Bair and O’Keefe 2. The latter procedures resulted in a further reduction of cells from our sample (due to the 6 required 5 trials per cell per condition; remaining cells n=118), but corroborated all previous analyses. We found a significantly higher choice probability for early SIDs than for late SIDs (median [25-75percentile] choice probabilityearly: 0.560 [0.486 0.619]; choice probabilitylate: 0.498 [0.421 0.565]; p<0.001, signed rank test). Moreover, the activity with early choices in preferred direction was significantly higher than the activity with early choices in anti-preferred direction, and higher than with late choices in either preferred direction or anti-preferred direction. Neither of the latter 3 differed significantly from one another (p<0.001, Kruskal-Wallis RMANOVA, for additional detail see table 1). Thus, eliminating trials where the eyeposition deviated by more than +/- 0.15° from the fixation spot, or eliminating trials where micro-saccades or drifts/pursuits occurred did not change the basic pattern of results. In addition to these controls we calculated whether the direction of micro-saccades (provided they occurred on a given trial) had an effect on neuronal activity. We first analysed the number of micro-saccades that occurred within the +/- 0.15 ° window, which we used for our post-hoc control. For micro-saccade detection we used published criteria2. Even within the small window of +/- 0.15 ° micro-saccades still occurred, but they were rare in numbers. In Monkey AR 9156/9850 (92.9%) trials for which neuronal activity was available were free of micro-saccades (during the time window from -700 to -100 ms before SID occurrence). In monkey CS this was the case for 19829/23467 (84.5%) of the trials for which neuronal activity was analysed. For trials during which micro-saccades occurred we constructed the saccade triggered averaged responses. This was done separately for saccades that caused retinal slip in preferred direction and saccades that caused retinal slip in anti-preferred direction. 7 The associated responses were largely flat, and no consistent difference in neuronal activity with these two types of retinal slip was found (see Supplementary Figure 1). From these controls we conclude that activity elicited by eye movements was not responsible for the differential activity reported in the main paper. Supplementary Figure 1: Relation of neuronal activity to the direction of micro-saccades. Saccade triggered average responses were constructed from all saccades that were within +/30 ° of the preferred direction of the neuron. If micro-saccades had a substantial X- and Ycomponent, the larger of the two was taken to generate the above eye-movement average. 8 Real world movement coordinates were reflected such that micro-saccades in preferred direction of a neuron yielded positive amplitudes (black line, dashed lines show s.e.m), while those in anti-preferred direction yielded negative amplitudes (grey line, grey shaded area shows s.e.m.). The average amplitude of the micro-saccades that occurred was ~0.15 °. The upper panel shows the neuronal activity associated with the two different types of microsaccades. The black line shows the activity that occurred with micro-saccades in preferred direction (i.e. the retinal slip was in anti-preferred direction, based on 1051 trials). Dashed lines show s.e.m.. The grey line shows the mean activity associated with micro-saccades in anti-preferred direction, i.e. with retinal slip in preferred direction (based on 1046 trials, grey shaded area shows s.e.m). There was no consistent increase of activity following retinal slip in preferred direction, or activity decrease following retinal slip in anti-preferred direction. Note that the same micro-saccade could contribute to saccades in preferred direction as well as to saccades in anti-preferred direction, as simultaneously recorded neurons could have opposite preferred direction. Hence the large similarity between the micro-saccades in preferred and those in anti-preferred direction. Fine rate correlation as a function of SID type We analysed whether fine rate correlation correlated with the choice type and direction for cell pairs provided at least 10 trials for each SID type occurred during recording. The median numbers of trials for the different SID types for the pairs from the 60° group (see main paper, n=46 pairs) were: PDearlySID: n=32 [min: 12, max: 119], APDearlySID: n=56 [min: 11, max: 128], PDlateSID: n=38 [min: 14, max: 91], APDlateSID: n=43 [min: 17, max: 139]). For the 120° group (n=68), the median number of trials for the different SID types was: PDearlySID: n=41 [min: 12, max: 187], APDearlySID: n=48 [min: 11, max: 153], PDlateSID: n=55 [min: 23, max: 131], APDlateSID: n=55 [min: 14, max: 139]). Supplementary Figure 2 shows the average neuronal fine rate correlation as a function of time for the 4 different SID types. Fine rate correlation 9 was measured in a sliding window of 200 ms width, starting at 800 ms (centred) before SID occurrence. Fine rate correlation strength was determined by calculating the correlation coefficient3 within a time window of -20 to 20 ms relative to the trigger spike. Supplementary Figure 2 shows that fine rate correlation increased at around 500 ms before SIDs in preferred direction, this increase peaked ~300ms before the SIDs and then returned to baseline. No such increase occurred for early SID in anti-preferred direction or any of the late SIDs. Supplementary Figure 2: Time resolved neuronal fine rate correlation (expressed by the correlation coefficient) for the 4 different SID types. Fine rate correlation was averaged across neurons that had a preferred direction within 0-120° of another (n=110). Fine rate correlation was calculated in a sliding window of 200 ms widths. It increased prior to SIDs in preferred direction shortly before the choice was indicated. Distributions of fine rate correlation coefficient for the different choice types Supplementary Figure 3 shows the distributions of the fine rate correlation coefficient for the different choice types. For ease of comparison pair-wise analysis of fine rate correlation strength for different choice types is shown. The figure shows 10 that fine rate correlation was stronger for the 60° group (additional detail is given in the main paper). Moreover, strength of fine rate correlation was also more consistent (i.e. better correlated) between different choice types for this group of neurons than for the 120° group (see insets of correlation coefficients and the associated p-values in each sub-panel). On top of the ongoing fine rate correlation (evident by the significant correlation between fine rate correlation values), choice type modulated the exact strength of fine rate correlation. The lack of correlation between choice types for the 120° cell group suggests that these neurons are less strongly coupled, less consistently coupled, and/or share either less common input. Moreover the absence of a significant effect of choice type on the strengths of fine rate correlation (see main paper) suggests that these neuronal pairs are also less modulated by ‘cognitive signals’, or alternatively, if fine rate correlation arises locally, contribute less to ‘cognitive decisions’. 11 Supplementary Figure 3: Distributions of fine rate correlation strength (correlation coefficient between simultaneously recorded neurons). Distributions are plotted pair-wise for different choice types to aid comparison. Upper plots (yellow histograms) show fine rate correlation 12 strength for neurons that shared a similar preferred direction (the 60° group), while lower plots (red histograms) show fine rate correlation strength between neurons of less similar preferred direction (120° group). Fine rate correlation was stronger for the 60° group than the 120° group. Moreover fine rate correlation seemed more consistent for the former group, evident by significant correlations between fine rate correlation coefficients across different choice types. Note, that despite overall larger consistency in the 60° group, strength of fine rate correlation significantly depended on SID type for the 60° group (larger for early SIDs in preferred direction), while this was not the case for the 120° group. In addition to similarity in preferred direction cooperation between neurons might be affected by other covariates. Another obvious candidate is the amount of receptive field overlap, as overlap determines the amount of common input and the likelihood of interconnectedness through lateral connections. To determine how receptive field overlap affects strength of fine rate correlation we analysed whether fine rate correlation depended on the receptive field overlap for each of the two groups (the 60° and the 120° group). Overlap of receptive field was expressed as the total overlap in receptive field area (deg2) divided by the sum of the individual receptive field areas. Each of the two groups (the 60° and the 120° group) was then subdivided into two further subgroups that were separated according to the median receptive field overlap. We refer to these subgroups as the ‘less overlap’ and ‘more overlap’ groups. The 60° group with more overlap had a mean overlap of 0.5678+/-0.1736, while the group with less overlap had a mean overlap of 0.0811+/- 0.1154. The 120° group with more overlap had a mean overlap in receptive field area of 0.5160+/0.2667, while the group with less overlap had a mean overlap of 0.0142+/- 0.0334. Overall we did not find a significant difference regarding receptive field overlap between the 60° and 120° group (p=0.1717, Wilcoxon rank sum test). However, the 13 two less overlap groups were significantly different (p=0.036, Wilcoxon rank sum test), while the groups with more overlap were not (p=0.2788, Wilcoxon rank sum test). We performed a 2-factor ANOVA to determine whether angular difference (60° vs. 120°), or the amount of receptive field overlap (upper half of overlap for each of the angular groups vs. lower half of overlap) had a significant effect on the strengths of neuronal fine rate correlation. We found a significant main effect of angular difference (p<0.0001, 2 Factor ANOVA), and a significant main effect of RF group (p=0.0391, 2 Factor ANOVA), but no interaction (p=0.2195, 2 Factor ANOVA). The average strength of neuronal fine rate correlation for both subgroups from both groups is shown in Supplementary Figure 4. Supplementary Figure 4: Strength of fine rate correlation as a function of similarity in preferred direction and receptive field overlap for the different SID types. Fine rate correlation was calculated as the neuronal correlation coefficient from spikes occurring from -500 to 100ms before the choice. The correlation coefficient was calculated over a window of -20 ms to 20 ms relative to the trigger spike. Bars show mean correlation coefficients, error bars show S.E.M. 14 For both subgroups from both groups we performed a repeated measurement ANOVA to determine whether SID time (early vs. late), direction (early vs. late), or an interaction between these two significantly affected the strength of fine rate correlation. Fine rate correlation was significantly affected by SID type for the 60° group with more receptive field overlap. Although fine rate correlation did not depend on SID direction (p=0.1325) or SID time (p=0.5955) alone, it depended significantly on the interaction between SID time and direction (p=0.0254, RM-ANOVA, n=21) for this group/subgroup. For the 60° group with less RF overlap neither of the individual factors determine the strength of fine rate correlation (time: p= 0.4473, direction: p= 0.7025), nor did the interaction (time*direction: p= 0.5067, RMANOVA, n=21). Neither of the two subgroups from the 120° group was significantly affected by SID time, SID direction or an interaction between the two (p>0.05, 2 factor RM-ANOVA, n=34 each subgroup). While this could be taken as evidence that fine rate correlation strength in the 120° group was unrelated to SID type, it can be seen from figure 8 B (main paper) and Supplementary Figure 4, that early SIDs in preferred direction were still preceded by the largest amount of fine rate correlation on average, while early SIDs in anti-preferred direction were preceded by the lowest average fine rate correlation. Splitting of the groups reduced sample size, and thus the strength of the effect had to be relatively larger to reach significance. This is probably why we found a significant effect of SID direction for the 120° group as a whole (see main paper), but not when splitting it according to receptive field overlap. These details aside, the analysis determined that the strength of fine rate correlation was largest prior to early SIDs in preferred direction, provided the neurons shared a 15 similar preferred direction and had more receptive field overlap. One would predict that precisely these neuron pairs would be best suited to contribute to directional decision in the absence of external visual motion. Neuronal fine rate correlation as a function of multi and single unit recording We recorded activity simultaneously from single units and from multi units. We recorded from a total of 30 single-single unit pairs whose preferred direction was less than 120° apart. The number of multi-multi unit pairs contributing to the paper is 35 and the number of single-multi unit pairs contributing to the data in the paper is 46. Given the relatively low pair numbers for each of these three subgroups we did not further separate them according to more similar preferred direction (<60° apart) and less similar preferred direction (60-120° apart), or according to receptive field size overlap. We find that the strength of fine rate correlation for all these pairs is largest for early SIDs in preferred direction, while it is lowest for early SIDs in anti-preferred direction, although this was only significant for the group of single-multi pairs (p=0.021, signed rank test). We found that single-single unit pairs exhibited the overall lowest fine rate correlation (mean correlation coefficient for early SIDs in preferred direction: 0.0397 +/-0.126), while single-multi unit (mean correlation coefficient for early SIDs in preferred direction: 0.0918 +/- 0.173) and multi-multi unit (mean correlation coefficient for early SIDs in preferred direction: 0.115 +/0.180) pairs exhibited fairly similar levels of fine rate correlation. The differences in fine rate correlation between the three groups were not significant (p>0.05, ANOVA on ranks), probably due to the relatively small sample size. 16 Coarse rate correlation as a function of SID type We performed similar analyses as described above for the coarse rate correlation data. Supplementary Figure 5 shows the distributions of coarse rate correlation for the different SID types separately for the two neuron groups (the 60° and the 120° group). In line with the data concerning neuronal fine rate correlation, coarse rate correlation was stronger for the 60° group than for the 120° group (for additional detail see main paper), and more consistent between different choice types (see insets of correlation coefficients and the associated p-values in each sub-panel). We performed a 2-factor ANOVA to determine whether angular difference (60° vs. 120°), or the amount of receptive field overlap (upper half of overlap for each of the angular groups vs. lower half of overlap) had a significant effect on the coarse rate correlation. We found a significant main effect of angular difference (p=0.0225, 2 Factor ANOVA), and a significant main effect of RF group (p=0.0455, 2 Factor ANOVA), and a significant interaction (p=0.0415, 2 Factor ANOVA). The average strength of coarse rate correlation for both subgroups from both groups is shown in Supplementary Figure 6. 17 Supplementary Figure 5: Distributions of coarse rate correlation from simultaneously recorded neurons. Distributions are plotted as pair-wise comparisons for different choice types to aid comparison. Upper plots (yellow histograms) show coarse rate correlation for neurons that shared a similar preferred direction (the 60° group), while lower plots (red 18 histograms) show coarse rate correlation between neurons of less similar preferred direction (120° group). Coarse rate correlation was stronger for the 60° group than the 120° group. Moreover coarse rate correlation seemed more consistent for the 60° group, evident by significant correlations between coarse rate correlation values across different choice types. Despite overall larger consistency, coarse rate correlation significantly depended on SID type for the 60° group (larger for early SIDs in preferred direction), while this was not the case for the 120° group. For both subgroups (more overlap vs. less overlap) from both groups (the 60° and the 120° group) we performed a repeated measurement ANOVA to determine whether SID time (early vs. late), direction (early vs. late), or an interaction between these two significantly affected coarse rate correlation. Coarse rate correlation was significantly affected by SID type for the 60° group with more receptive field overlap. Although it did not depend on SID direction (p=0.2484) or SID time (p=0.5091) alone, it depended significantly on the interaction between SID time and direction (p=0.0493, RM-ANOVA, n=21) for this group/subgroup. For the 60° group with less RF overlap neither of the individual factors determined the strength of coarse rate correlation (time: p= 0.7071, direction: p= 0.8037). There was a trend that an interaction between SID time and direction influenced strength of coarse rate correlation, but it did not reach significance (time*direction: p= 0.0797, RM-ANOVA, n=21). For the subgroup with less receptive field overlap from the 120° group there was a nonsignificant trend that SID direction (p=0.0560, RM-ANOVA, n=34) affected coarse rate correlation, while neither SID time (p=0.4444, RM-ANOVA, n=34), nor an interaction between direction and time (p=0.6030, RM-ANOVA, n=34) showed a similar trend. SID direction significantly affected coarse rate correlation in the 120° with more receptive field overlap (p=0.0246, RM-ANOVA, n=34), while SID time 19 (p=0.3706, RM-ANOVA, n=34) had no significant effect on coarse rate correlation for this group, nor was there a significant interaction between SID direction and time (p=0.7878, RM-ANOVA, n=34). The analysis showed that coarse rate correlation was generally strongest before early SIDs in preferred direction, provided the neurons had more receptive field overlap. Similarity in preferred direction also affected strength of coarse rate correlation, but its relation to SID type was less consistent. This is particularly evident by the strength of coarse rate correlations as a function of SID type for neurons that have neither a similar preferred direction nor have substantial amount of receptive field overlap (see the black bars in Supplementary figure 6 B). In the context of the current paper such a result makes sense, if neurons do not code for the same preferred direction and do not represent the same part of the visual field, their possible combined contribution to directional decisions in the absence of visual stimuli is not immediately obvious. Supplementary Figure 6: Strength of coarse coarse rate correlation as a function of similarity in preferred direction and receptive field overlap for the different SID types. Coarse rate 20 correlations were calculated from spikes occurring within -500 to -100ms before the choice. Bars show mean coarse rate correlation, error bars show S.E.M. 1. 2. 3. Thiele, A., Distler, C. & Hoffmann, K. P. Decision-related activity in the macaque dorsal visual pathway. Eur J Neurosci 11, 2044-58. (1999). Bair, W. & O'Keefe, L. P. The influence of fixational eye movements on the response of neurons in area MT of the macaque. Vis Neurosci 15, 779-86. (1998). Bair, W., Zohary, E. & Newsome, W. T. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J Neurosci 21, 1676-97. (2001). 21