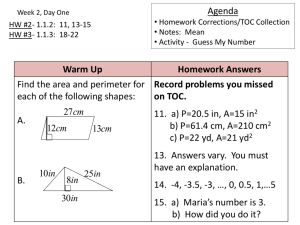
Chapter 10
Energy
Section 10.1
The Nature of Energy
Energy
•
Energy is anything that has the ability to
do work or produce heat.
All chemical and physical changes
result in the matter changing energy.
Return to TOC
Copyright © Cengage Learning. All rights reserved
2
Section 10.1
The Nature of Energy
Energy
•
•
Potential energy – stored energy, due to
position or composition.
Kinetic energy – energy of motion, energy
that is being transferred from one object
to another.
Return to TOC
Copyright © Cengage Learning. All rights reserved
3
Section 10.1
The Nature of Energy
Law of Conservation of Energy
•
Energy can be converted from one form to
another, but can be neither created nor
destroyed.
The energy of the universe is
constant—first law of thermodynamics.
Return to TOC
Copyright © Cengage Learning. All rights reserved
4
Section 10.1
The Nature of Energy
Energy
Return to TOC
Copyright © Cengage Learning. All rights reserved
5
Section 10.2
Temperature and Heat
Temperature
•
A measure of the random motions of the components
of a substance.
Return to TOC
Copyright © Cengage Learning. All rights reserved
6
Section 10.2
Temperature and Heat
Heat
•
A flow of energy between two objects due to a
temperature difference between the objects.
Return to TOC
Copyright © Cengage Learning. All rights reserved
7
Section 10.3
Exothermic and Endothermic Processes
•
•
System – part of the universe on which we
wish to focus attention.
Surroundings – include everything else in
the universe.
Return to TOC
Copyright © Cengage Learning. All rights reserved
8
Section 10.3
Exothermic and Endothermic Processes
•
Endothermic Process:
Heat flows into a system.
Absorb energy from the surroundings.
Surroundings
reaction
Return to TOC
Copyright © Cengage Learning. All rights reserved
9
Section 10.3
Exothermic and Endothermic Processes
•
Exothermic Process:
Energy flows out of the system.
Energy gained by the surroundings is
equal to the energy lost by the system.
Surroundings
reaction
Return to TOC
Copyright © Cengage Learning. All rights reserved
10
Section 10.3
Exothermic and Endothermic Processes
Changes in State
Return to TOC
Copyright © Cengage Learning. All rights reserved
11
Section 10.3
Exothermic and Endothermic Processes
Concept Check
Classify each process as exothermic or
endothermic. Explain. The system is
underlined in each example.
a)Your hand gets cold when you touch ice.
b)The ice gets warmer when you touch it.
c)Water boils in a kettle being heated on a stove.
d)Water vapor condenses on a cold pipe.
e)Ice cream melts.
Return to TOC
Copyright © Cengage Learning. All rights reserved
12
Section 10.5
Measuring Energy Changes
•
The common energy units for heat are the
calorie and the joule.
calorie (cal) – the amount of energy
(heat) required to raise the temperature
of one gram of water 1oC.
Joule (J) – 1 calorie = 4.184 joules
Return to TOC
Copyright © Cengage Learning. All rights reserved
13
Section 10.5
Measuring Energy Changes
Energy (Heat) Required to Change the Temperature of a
Substance Depends On:
1. The amount of substance being heated
(number of grams).
2. The temperature change (number of
degrees).
3. The identity of the substance.
Return to TOC
Copyright © Cengage Learning. All rights reserved
14
Section 10.5
Measuring Energy Changes
Specific Heat Capacity
•
•
Heat capacity is the amount of heat a
substance must absorb to raise its
temperature by 1 °C.
Specific heat = heat capacity of 1 gram of
the substance.
Return to TOC
Copyright © Cengage Learning. All rights reserved
15
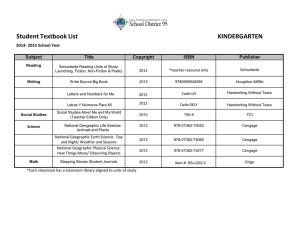
Section 10.5
Measuring Energy Changes
Specific Heat Capacities of Some Common Substances
Return to TOC
Copyright © Cengage Learning. All rights reserved
16
Section 10.5
Measuring Energy Changes
Energy Required for a Reaction or Process:
•
Energy (heat) required, Q = s × m × ΔT
Q = energy (heat) required (J)
s = specific heat capacity (J/°C·g)
m = mass (g)
ΔT = change in temperature (°C)
Return to TOC
Copyright © Cengage Learning. All rights reserved
17
Section 10.5
Measuring Energy Changes
Example
Calculate the amount of heat energy (in
joules) needed to raise the temperature of
6.25 g of water from 21.0°C to 39.0°C.
Return to TOC
Copyright © Cengage Learning. All rights reserved
18
Section 10.5
Measuring Energy Changes
Exercises
1. A sample of pure iron requires 142 cal of energy to
raise its temperature from 23ºC to 92ºC. What is
the mass of the sample? (The specific heat
capacity of iron is 0.45 J/gºC.)
2. A 100.0 g sample of water at 90.°C is added to a
500.0 g sample of water at 10.°C. Calculate the
final temperature of the water.
Return to TOC
Copyright © Cengage Learning. All rights reserved
19
Section 10.5
Measuring Energy Changes
Summary of Topics: Chapter 10
• Energy
• Endothermic, exothermic
– Phase changes
• Heat Capacity
Return to TOC
Copyright © Cengage Learning. All rights reserved
20