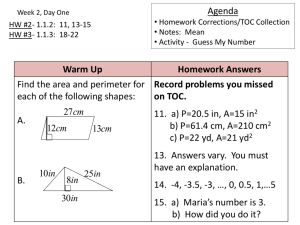
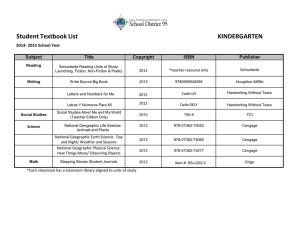
Chapter 2
Measurements and
Calculations
Section 2.1
Scientific Notation
Measurement
• Quantitative observation.
• Has 2 parts – number
and unit.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.1
Scientific Notation
• Technique used to express very large or very
small numbers.
• Expresses a number as a product of a
number between 1 and 10 and the
appropriate power of 10.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.1
Scientific Notation
Using Scientific Notation
• The number of places the decimal point is
moved determines the power of 10. The
direction of the move determines whether the
power of 10 is positive or negative.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.1
Scientific Notation
Using Scientific Notation
• If the decimal point is moved to the left, the
power of 10 is positive.
• If the decimal point is moved to the right, the
power of 10 is negative.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.1
Scientific Notation
1. The World’s population is estimated to be
7,187,000,000 people. Express this number in
scientific notation.
2. Express the following numbers in scientific
notation: 0.0000671; 72.
3. Express the following numbers in standard
notation: 2.598 x 10-7; 9.5 x 104.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.2
Units
Nature of Measurement
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.2
Units
The Fundamental SI Units
Physical Quantity
Mass
Length
Time
Temperature
Electric current
Amount of substance
Name of Unit
kilogram
meter
second
kelvin
ampere
mole
Abbreviation
kg
m
s
K
A
mol
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.2
Units
Prefixes Used in the SI System
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.3
Measurements of Length, Volume, and Mass
Length
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.3
Measurements of Length, Volume, and Mass
Volume
•
Measure of the amount
of 3-D space occupied
by a substance.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.3
Measurements of Length, Volume, and Mass
Mass
•
Measure of the amount
of matter present in an
object.
Weight
•
Measure of the gravitational
pull on an object.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.3
Measurements of Length, Volume, and Mass
Concept Check
Choose the statement(s) that contain improper
use(s) of commonly used units (doesn’t make
sense)?
A gallon of milk is equal to about 4 L of milk.
A 200-lb man has a mass of about 90 kg.
A basketball player has a height of 7 m tall.
A nickel is 6.5 cm thick.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.4
Uncertainty in Measurement
Measurement of Length Using a Ruler
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.4
Uncertainty in Measurement
•
•
•
A digit that must be estimated is called
uncertain.
A measurement always has some degree of
uncertainty.
Record the certain digits and the first uncertain
digit (the estimated number).
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.5
Significant Figures
Rules for Counting Significant Figures
Nonzero integers always count as significant
figures.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.5
Significant Figures
Rules for Counting Significant Figures
1. Leading zeros are zeros that precede all the nonzero
digits. These do not count as significant figures.
2. Captive zeros are zeros between nonzero digits. These
always count as significant figures.
3. Trailing zeros are zeros at the right end of the number.
They are significant only if the number contains a
decimal point.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.5
Significant Figures
Exponential Notation
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.5
Significant Figures
Rules for Counting Significant Figures
Exact numbers have an infinite number of
significant figures.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.5
Significant Figures
Rules for Rounding Off
1. If the digit to be removed is less than 5, the
preceding digit stays the same. If the digit to
be removed is equal to or greater than 5, the
preceding digit is increased by 1.
2. In a series of calculations, carry the extra digits
through to the final result and then round off.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.5
Significant Figures
Significant Figures in Mathematical Operations
1. For multiplication or division, the number of
significant figures in the result is the same as
that in the measurement with the smallest
number of significant figures.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.5
Significant Figures
Significant Figures in Mathematical Operations
2. For addition or subtraction, the limiting term is
the one with the smallest number of decimal
places.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.5
Significant Figures
Concept Check
You have water in each graduated
cylinder shown. You then add both
samples to a beaker (assume that
all of the liquid is transferred).
How would you write the number
describing the total volume?
What limits the precision of the
total volume?
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.5
Significant Figures
1. An impossibly regular, paved walkway mysteriously
appears overnight; leading out of Seattle. Careful
measurement shows this walkway to be 15,432 meters
long and 0.42 meters wide. To the correct number of
significant figures, what area is covered by walkway?
How would this number change if the walkway were
0.41 meters wide? 0.43 meters wide?
2. By the next morning, this walkway has grown 0.42
meters. To the correct number of significant figures,
how long is it now?
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.6
Problem Solving and Dimensional Analysis
•
Use when converting a given result from one
system of units to another.
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.5
Significant Figures
1. A golfer putted a golf ball 6.8 ft across a green. How
many inches does this represent? How many
centimeters?
2. What is the volume of a 1.25 gallon jug in cubic
centimeters? Cubic inches? (1 L = 1.057 qts)
3. An iron sample has a mass of 4.50 lb. What is the mass
of this sample in grams? (1 kg = 2.2046 lbs)
4. If an oxygen molecule is moving at 4.78 x 104 cm/s,
what is its speed in mi/hr?
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.7
Temperature Conversions: An Approach to Problem Solving
Three Systems for Measuring Temperature
•
•
•
Fahrenheit
Celsius
Kelvin
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.7
Temperature Conversions: An Approach to Problem Solving
The Three Major Temperature Scales
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.7
Temperature Conversions: An Approach to Problem Solving
Converting Between Scales
•
Converting between scales
TK T C + 273
TC
T F
32
1.80
T C TK 273
T F 1.80 T C + 32
1. The normal body temperature for a dog is
approximately 102oF. What is this equivalent to on
the Kelvin temperature scale?
2. At what temperature does C = F?
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.8
Density
•
Mass of substance per unit volume of the
substance.
mass
Density =
volume
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.8
Density
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.8
Density
Measuring the Volume of a Solid Object by Water Displacement
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.8
Density
Example
1. A certain mineral has a mass of 17.8 g and a volume
of 2.35 cm3. What is the density of this mineral?
2. What is the mass of a 49.6 mL sample of a liquid,
which has a density of 0.85 g/mL?
3. Copper has a density of 8.96 g/cm3. If 75.0 g of
copper is added to 50.0 mL of water in a graduated
cylinder, to what volume reading will the water level
in the cylinder rise?
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.8
Density
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.8
Density
Return to TOC
Copyright © Cengage Learning. All rights reserved
Section 2.8
Density
Summary of Topics: Chapter 2
•
•
•
•
•
•
•
Significant figures
Scientific notation
Metric units
Measured numbers, exact numbers
Dimensional analysis (conversions)
Temperature
Density
Return to TOC
Copyright © Cengage Learning. All rights reserved