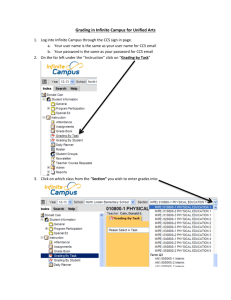
Supplemental 1
advertisement

Supplemental 1 All CCS calibrations and measurements were performed, in part, by our opensource software pepCCScal. All calculations are based off methods described previously [1, 2]. Briefly, an empirically modified version of the Mason-Schamp equation is used: 𝑧𝑒 18𝜋 Ω = 16 [𝐾 𝑏 1 1 1/2 ( + 𝑀) ] 𝑇 𝑚 760 𝑇 1 𝑃 273.15 𝑁 𝐴𝑡𝐷 𝐵 (1) The variable Ω is the CCS, z is the ion charge state, e is the fundamental charge, K b is Boltzmann’s constant, T is temperature, m is the mass of the analyte, M is the mass of the drift gas, P is the pressure, N is the buffer gas number density, and tD is the ion’s drift time or arrival time. The constants A and B result from the non-uniformity of the electric field and must be determined by calibration. The CCS can be normalized by reduced mass and charge state (Ω’), and all other variables can be collapsed into a single term (A’) for the following reduced equation: Ω′ = 𝐴′𝑡𝐷 𝐵 (2) The user defines the calibration set with a 3-column csv file. Column A is the calibrant peptide sequence, column B is the calibrant charge, and column C is the calibrant CCS. This file is selected by the “Select Cal Standards” button. After IM-MS acquisition of the calibrants, the file is opened in DriftScope and “Detect Peaks” is selected from the Peak Detection menu. The non-chromatographic, lockspray-corrected peaklist is then created and exported as a csv. This file is selected from the “Select Cal Input” button. To create the calibration, pepCCScal calculates the monoisotpic m/z of the user-defined calibrants and searches the lockspray-corrected m/z from the DriftScope peak list csv (column C). If a match within the user-defined ppm threshold is found, pepCCScal assigns the corrected and centroided drift time (tD) (column H) to that calibrant. When the all observed calibrants are found, pepCCScal begins the two-part calculation. First, it calculates the slope of a best-fit linear regression for ln (Ω’) versus ln (tD), with Ω’ being the calibrant CCS normalized for mass and charge. The slope of this log plot is the B term from equations 1 and 2. Next, doubly corrected tD are calculated for each calibrant: 1 1 1/2 𝑡𝐷′′ = 𝑧 (𝑚 + 𝑀) 𝑡𝐷 𝐵 (3) The calibration is completed by calculating the slope and y-intersection of a best-fit linear regression for Ω versus tD’’. The mean error, RMSE, and R2 for the calibration are provided in the output file. This equation is used to calculate unknown CCS measured at the same wave height and wave velocity. For CCS measurement in direct infusion experiments, the analyte is acquired after calibration. This acquisition is also opened in DriftScope to create a lock-spray corrected, non-chromatographic peak list which is exported as a csv. The pepCCScal program can search for ions based on a list of peptide sequences and charges, or the user can manually enter mass and charge values to look for. The best-fit equation from the calibration output csv is used to calculate the CCS of these ions. For HDMSE CCS profiling, DriftScope is not required to make a peak list. The Final Peptide csv from PLGS output contains the centroid tD of all identified peptides. However, this csv contains tD in bins, not milliseconds, and so polyalanine calibration must also be done in bins. The pepCCScal program uses the tD from the Final Peptide csv (column BE) to calculate CCS. Microsoft Visual C# was used to write pepCCScal. The program and source code can be obtained for free by contacting the corresponding author. The mean polyalanine calibration error, the discrepancy of known polyalanine CCS values from their CCS, was (0.44 ± 0.03) % for high voltage acquisitions and (0.34 ± 0.03) % for low voltage acquisitions. Table S1 Table S2 Figure S1. LC-HDMSE chromatograms total peptide IDs from approximately 1.5 µg of tryptic total protein from yeast lysate. The same sample was acquired under (a) low and (b) high transmission voltages. Figure S2. 940 µM BK3+ was acquired with EDC target enhancement at the lowest transmission voltages we were able to achieve: 2.0 kV spray voltage, 1.5 V extraction cone, trap bias 33 V, and He cell DC 10 V. The cone voltage was set to different values to apply activation energy. Arrival time profiles at 20 V cone voltage and below were nearly identical. Figure S3. Plots of CCS versus m/z for tryptic K562 peptides, LysN yeast peptides, and LysC yeast peptides. These plots only use first-pass PepFrag1 PLGS identifications and omit miscleavages. Supplemental References 1. 2. Ruotolo B. T., Benesch J. L., Sandercock A. M., Hyung S. J., Robinson C. V.: Ion mobility-mass spectrometry analysis of large protein complexes. Nature protocols, 3,1139-1152 (2008) Smith D. P., Knapman T. W., Campuzano I., Malham R. W., Berryman J. T., Radford S. E., Ashcroft A. E.: Deciphering drift time measurements from travelling wave ion mobility spectrometry-mass spectrometry studies. European journal of mass spectrometry, 15,113-130 (2009)