What would you predict for the molar heat capacity of... , based on its structure? (A) C
advertisement
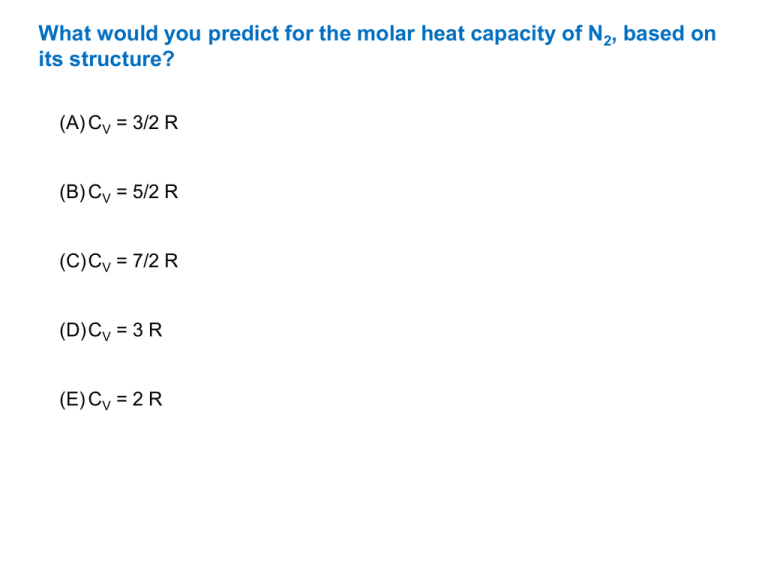
What would you predict for the molar heat capacity of N2, based on its structure? (A) CV = 3/2 R (B) CV = 5/2 R (C) CV = 7/2 R (D) CV = 3 R (E) CV = 2 R What would you predict for the molar heat capacity of N2, based on its structure? (A) CV = 3/2 R (B) CV = 5/2 R (C) CV = 7/2 R because it is a diatomic (i.e. linear) molecule; 2 atoms (3N-5/2)R = 7/2 R (D) CV = 3 R (E) CV = 2 R We just established hat the molar heat capacity of N2, should be 7/2R, which is 29.1 J mol-1 K-1. The experiment gives us 20.8 J mol-1 K-1 at 25 C. Why the big difference? (A) Because N2 is not an ideal gas. (B) Because some of the degrees of freedom are not active at this temperature. (C) Because we are too close to the critical point at 25 C. We just established hat the molar heat capacity of N2, should be 7/2R, which is 29.1 J mol-1 K-1. The experiment gives us 20.8 J mol-1 K-1 at 25 C. Why the big difference? (A) Because N2 is not an ideal gas. (B) Because some of the degrees of freedom are not active at this temperature. … to be exact, the vibrational degree of freedom! (C) Because we are too close to the critical point at 25 C.