Using Biochar as a Soil Amendment for Sustainable Agriculture
advertisement

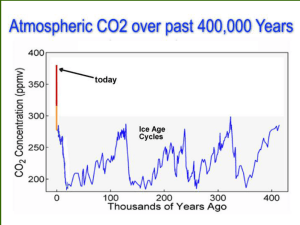
Using Biochar as a Soil Amendment for Sustainable Agriculture W. Zheng B.K. Sharma K. Rajagopalan Biochar Symposium Illinois Sustainable Technology Center (ISTC) June 9, 2011 Using Biochar as a Soil Amendment for Sustainable Agriculture Grant #: SA 09-37 (2009-2010) Sustainable Agriculture Grant Program by Illinois Department of Agriculture Illinois Sustainable Technology Center (ISTC) Project Goal The objective of this project was to examine the potential use of biochar as a soil amendment in a typical corn field in Illinois as part of a larger goal of promoting sustainable agricultural practice. To achieve this goal, three tasks were undertaken in the project: Biochar production and characterization: Biochar production through a low-temperature slow pyrolysis technique from a variety of waste biomass. Removal of nutrients by biochar: The sorption kinetics and mechanisms of NH4+ and PO43- removal by biochar were investigated. Field trial to demonstrate the efficacy of biochar as a simple soil amendment as measured by crop yields and lowered fertilizer use in Illinois, which attempted to investigate if the use of biochar as a soil amendment could reduce chemical fertilizer use while at the same time maintaining or increasing crop yields. Feedstock The feedstocks used for biochar production in this study focused on three kinds of waste biomass: Agricultural residues corn cobs corn stover; Yard wastes walnut shells and wood chips; By-products from bioenergy defatted dried distiller grains (DDGs) Pyrolysis Syngas H2, CO, CO2 Bio-oil Waste Biomass Pyrolysis Pyrolysis is a most common thermochemical conversion process where biomass is heated in the absence of oxygen to yield a series of bioproducts: syngas; bio-oil; and biochar. Biochar Schematic Diagram for Biochar Production in a Slow Pyrolyzer . ISTC Sustainable Biochar Effect of Selected Feedstocks and Pyrolysis Conditions on Yields of Bioproducts Biochar Feedstock ZW-1 Corn cob 32.2 % 45.6 % Syngas (%) 22.2% ZW-2 Corn stover 39.0 % 42.8 % 18.2 % ZW-3 Defatted DDG 45.8 % 40.3 % 14.9 % ZW-4 Pine cone 38.0 % 44.4 % 17.6 % America chestnut shell ZW-6-1* Wood chip 42.2 % 45.6 % 12.2 % 35.0 % 42.0 % 23.0 % ZW-6-2* Wood chip 35.0 % 42.1 % 22.9 % ZW-6-3* Wood chip 35.1 % 42.1 % 22.8 % ZW-5 Biochar (%) Bio-oil (%) The yields of three bio-products produced from selected feedstocks under oxygen-limited condition for 60 min at 400 oC. *ZW-6-1, 2, and 3 refer to the feedstock pyrolyzed under 0, 2, and 5 L/min nitrogen flow. Effect of Pyrolysis Temperature on the Yields of Bioproducts 60 Biochar Product Yield (%) 50 Bio-oil 40 30 Syngas 20 10 250 300 350 400 450 500 o Pyrolysis Temperature ( C) 550 Biochar Characterization on Physicochemical Properties Feedstock Pyrolysis SSA %C %H %N %O (O+N)/C O/C H/C Temperature (m2/g) % % Moisture Ash Corn cob 250 OC 1.86 61.16 4.96 0.82 27.82 0.353 0.341 0.973 1.32 3.92 Corn cob 300 OC 2.42 70.54 4.19 0.81 19.06 0.213 0.203 0.713 1.3 4.1 Corn cob 350 OC 3.36 72.92 3.79 0.79 16.86 0.183 0.173 0.624 1.29 4.35 Corn cob 400 OC 4.70 75.23 3.37 0.82 14.11 0.150 0.141 0.538 1.35 5.12 Corn cob 450 OC 7.79 77.84 2.95 0.86 11.45 0.120 0.110 0.455 1.35 5.55 Corn cob 500 OC 17.08 80.85 2.5 0.97 8.87 0.093 0.082 0.371 1.25 5.56 Corn cob 550 OC 30.57 82.62 2.25 0.84 7.43 0.076 0.067 0.327 1.28 5.58 Wood pellet 750 OC 105.3 81.99 1.14 0.52 3.04 0.033 0.028 0.167 4.56 8.75 Wood chip 450 OC 12.96 70.44 2.67 1.11 13.86 0.161 0.148 0.455 1.69 10.23 Defatted DDG 400 OC 1.98 64.43 3.76 7.44 10.14 0.217 0.118 0.700 1.45 12.78 Corn stover 400 OC 4.69 55.98 3.4 0.43 18.16 0.250 0.243 0.729 1.28 20.75 Pine cone 400 OC 17.92 73.88 3.21 1.33 15.31 0.171 0.155 0.521 1.32 4.95 91.1 0.9 0.28 5.71 0.050 0.047 0.119 1.12 0.89 Activated carbon 988.4 Project Goal The objective of this project was to examine the potential use of biochar as a soil amendment in a typical corn field in Illinois as part of a larger goal of promoting sustainable agricultural practice. To achieve this goal, three tasks were undertaken in the project: Biochar production and characterization: Biochar production through a lowtemperature slow pyrolysis technique from a variety of waste biomass. Removal of nutrients by biochar: The sorption kinetics and mechanisms of NH4+ and PO43- removal by biochar were investigated. Field trial to demonstrate the efficacy of biochar as a simple soil amendment as measured by crop yields and lowered fertilizer use in Illinois, which attempted to investigate if the use of biochar as a soil amendment could reduce chemical fertilizer use while at the same time maintaining or increasing crop yields. Sorption Capacities of NH4+ and PO43- on Selected Biochars and a Commercial Activated Carbon Ammounium ion Cs (mmol/g) 0.3 PO43- 0.25 0.2 0.15 0.1 Phosphate ion Cs (mmol/g) 0.05 0.35 0 0.3 0.25 0.2 0.15 0.1 0.05 0 NH4+ 1.0 PO43o Biochar produced from wood chip at 750 C o Biochar produced from wood chip at 450 C 0.9 0.8 0.7 0 10 20 30 Time (hrs) NH4+ 40 50 Phosphate Ion Concentration (mmol/L) Ammonium Ion Concentration (mmol/L) Sorption Kinetics 1.0 0.9 o Biochar produced from wood chip at 750 C o Biochar produced from wood chip at 450 C 0.8 0.7 0.6 0.5 0.4 0.3 0 10 20 30 Time (hrs) 40 50 Sorption Kinetics To investigate the controlling mechanisms of sorption processes, e.g., mass transfer and chemical reaction, the data obtained from this study were analyzed using two kinetic equations: the pseudofirst order equation and the pseudo-second order equation: where Qe and Qt are the amounts of nutrients sorbed (mmol/g) at equilibrium and at time t (h), k1 and k2 are sorption rate constants of pseudo-first order and pseudo-second order, respectively. The fit of these two models was checked by the linear plot of log (Qe-Qt) versus t and t/Qt versus t, respectively, and by comparison to the regression coefficients for each expression. Sorption Kinetics Pseudo-first order NH4+ PO43- k1 Qe (cal) (h-1) (mmol/g) Biochar-750 0.115 7.87 x 10-3 Biochar-450 0.105 Biochar-750 Biochar-450 Pseudo-second order R2 R2 k2 Qe (cal) (mmol/g)-1h-1 (mmol/g) 0.959 68.6 2.44 x 10-2 0.999 1.41 x 10-2 0.992 28.9 3.14 x 10-2 0.995 0.119 6.05 x 10-2 0.984 1.23 8.61 x 10-2 0.932 0.097 5.79 x 10-2 0.990 0.013 4.81 x 10-1 0.006 Pseudo-first order and pseudo-second order sorption rate constants of NH4+ and PO43- on two selected biochars Sorption Isotherms of NH4+ and PO43- on Selected Biochars NH4 + PO4 0.4 3- NH4 0.20 Cs (mmol/g) 0.3 + o Biochar produced from wood pellet at 750 C o Biochar produced from wood chip at 450 C 0.1 0.15 Cs (mmol/g) 0.2 0.0 0 0.10 5 10 15 20 Ce (mmol/L) o Biochar produced from wood pellet at 750 C o Biochar produced from wood chip at 450 C 0.05 PO43- 0.00 0 5 10 15 20 Ce (mmol/L) 25 30 25 30 Sorption Isotherm Freundlich isotherm Langmuir isotherm log Cs = log Kf + 1/n log Ce Ce/Qe = 1/(bQ0) + Ce/Q0 Kf 1/n R2 (mmol/g) (mmo/L)-n NH4+ PO43- Q0 b (mmol/g) (L/mmol) R2 Biochar-750 2.56 x 10-2 0.650 0.971 0.246 0.108 0.933 Biochar-450 4.12 x 10-2 0.516 0.981 0.234 0.190 0.974 Biochar-750 5.74 x 10-2 0.795 0.895 0.576 0.140 0.835 Biochar-450 3.60 x 10-2 0.933 0.873 0.787 0.047 0.270 Removal Mechanisms of Phosphate & Ammonium by Biochar Precipitation process Ca2+ + PO43- +.xH20 → Ca3(PO4)2.xH20 Biochar no washing mg/L mg/L < 0.6 < 0.6 Potassium* 7.7 15 Calcium* 2.8 3.8 Beryllium < 0.002 < 0.002 0.089 0.094 Magnesium 1.5 2.3 Aluminum 0.075 0.084 2.0 1.0 Titanium 0.0027 0.0022 Vanadium < 0.001 < 0.001 Chromium 0.0032 0.0033 Manganese 0.0077 0.0055 < 0.1 0.11 -21.9±3.8 -22.3±6.9 Analyte Units Sodium* 50 Phosphate removal (%) Biochar after DI H2O washing 40 30 Boron 20 10 Silicon 0 Biochar no washing Biochar with DI H2O washing Surface sorption Biochar with negatively charged surface Iron* Zeta potential (ζ) Sorption Mechanism of Phosphate by Biochar Ca2+ + PO43- +.xH20 → Ca3(PO4)2.xH20 Ca3(PO4)2.xH20 XAD patterns of before and after PO43- adsorption by biochar Project Goal The objective of this project was to examine the potential use of biochar as a soil amendment in a typical corn field in Illinois as part of a larger goal of promoting sustainable agricultural practice. To achieve this goal, three tasks were undertaken in the project: Biochar production and characterization: Biochar production through a lowtemperature slow pyrolysis technique from a variety of waste biomass. Removal of nutrients by biochar: The sorption kinetics and mechanisms of NH4+ and PO43- removal by biochar were investigated. Field trial to demonstrate the efficacy of biochar as a simple soil amendment as measured by crop yields and lowered fertilizer use in Illinois, which attempted to investigate if the use of biochar as a soil amendment could reduce the application rates of chemical fertilizer while at the same time maintaining or increasing crop yields. 2010 Biochar Field Experiment Design 90 Feet and 36 rows 60 Feet No Fertilizer Half Fertilizer Full Fertilizer 10 feet x 0.66 feet used for biochar treatments No Fertilizer Half Fertilizer Full Fertilizer Biochar Application in a Corn Field Biochar Application in Corn Field Standard corn growing practices ISTC Biochar Website http://www.istc.illinois.edu/research/biochar.cfm Biochar Application in Corn Field Yeilds of Corn Crop (bushel/acre) 280 240 No Fertilizer 50% Fertilizer 100% Fertilizer 200 160 120 80 40 0 Control Biochar-B Biochar-A 0 50 % 100 % No Biochar 139.3 a 174.3 a 173.0 a Biochar-A 164.6 b 213.7 b 239.8 b Biochar-B 170.9 b 194.2 b 201.3 a Nitrogen Fertilizer Selected Soil Properties Before and After Experiments Treatments Soil Organic Matter (%) Neutral Ammonium Acetate (exchangeable) P1 mg/kg P1 mg/kg K mg/kg Mg mg/kg Ca mg/kg pH Cation Exchange Capacity (CEC) meq/100g Phosphorus Nitrate-N mg/kg Before experiment No fertilizer and no biochar No fertilizer with biochar-A No fertilizer with biochar-B 3.2 3.6 4.1 15 19 20 22 23 28 206 326 218 345 274 349 1953 1808 2046 5.8 5.7 5.7 16.3 15.4 17.3 19 39 35 50% fertilizer and no biochar 4.2 19 32 180 438 2340 5.6 20.7 32 50% fertilizer with biochar-A 4.7 27 42 358 406 2474 5.6 19.0 33 50% fertilizer with biochar-B 4.0 21 31 251 362 1986 5.2 20.8 67 100% fertilizer and no biochar 4.6 12 17 172 527 2617 6.0 21.1 34 100%fertilizer with biochar-A 4.6 22 43 195 449 2422 5.9 19.7 75 100% fertilizer with biochar-B After experiment (at harvest) No fertilizer and no biochar No fertilizer with biochar-A No fertilizer with biochar-B 3.3 15 24 149 345 1878 5.4 17.6 74 3.9 5.0 4.5 20 33 19 30 46 31 198 259 166 310 318 329 1906 2105 1915 5.4 6.1 5.8 15.2 16.1 20.1 21 12 21 50% fertilizer and no biochar 4.5 22 38 176 366 2234 5.5 18.3 32 50% fertilizer with biochar-A 5.5 40 74 342 313 2298 6.2 19.0 29 50% fertilizer with biochar-B 4.8 29 47 179 324 2077 6.1 21.3 25 100% fertilizer and no biochar 4.2 14 21 175 456 2398 5.8 17.7 58 100%fertilizer with biochar-A 5.1 35 60 239 403 2308 6.5 22.6 13 100% fertilizer with biochar-B 4.9 27 39 221 376 2247 5.9 20.9 56 Colleagues Dr. Sharma, B.K. John Scott Dr. Li, X. Christie Teausant Nancy Holm Brent Panno Dr. Rajagopanlan, K. Dr. Kulkarni, M. Dr. Marlin, J. Monte Wilcoxon Joe Pickowitz Ed Zaborski Acknowledgments This study is being supported by Illinois Department of Agriculture’s Sustainable Agriculture Grant Program Questions Stay on the Stage