CBIT Application
advertisement

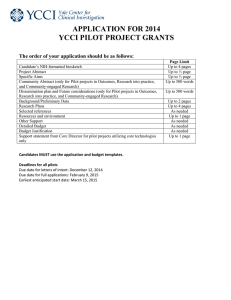
APPLICATION FOR 2014 Pilot Projects Utilizing the Center for Biomedical and Interventional Technology (CBIT) The order of your application should be as follows: Candidate’s NIH-formatted biosketch Project Abstract Specific Aims Unmet Clinical Need and Market Proposed Technical Solution and Background/Preliminary Data Research and Development Plan – How will you execute on the specific aims? Proposed Product Pathway – What proposed product will result from this work? What is the regulatory path? How will a doctor and hospital approach value analysis for the product? Commercialization – how will this award substantially enhance strength of an application for SBIR, STTR, or similar external funding? Description of the interdisciplinary nature of the project, the structure of the team, and plans for collaboration Selected references Resources and environment Other Support Detailed Budget Budget Justification Page Limit Up to 4 pages Up to ½ page Up to ½ page Up to 1 page Up to 4 pages Up to 4 pages Up to 2 pages Up to 1 page Up to 2 pages As needed Up to 1 page As needed As needed As needed Candidates MUST use the application and budget templates. Pilot Projects Utilizing the Center for Biomedical and Interventional Technology (CBIT) deadlines: Pre-application: December 12, 2014 Earliest anticipated start date for pre-application: January 1, 2015 Full application: June 19th, 2015 Earliest anticipated start date: July 31st, 2015 Version 5 13 15 Insert Candidate’s NIH-formatted Biosketch: Templates and samples can be found at: http://grants.nih.gov/grants/funding/phs398/biosketch.doc http://grants.nih.gov/grants/funding/phs398/biosketchsample.doc Version 5 13 15 Research Plan Project Abstract (Limited to ½ page of text): Specific Aims (Limited to ½ page of text): Version 5 13 15 Unmet Clinical Need and Market (Limited to 1 page including text and graphics): Version 5 13 15 Proposed Technical Solution and Background/Preliminary Data (Limited to 4 pages including text and graphics): Version 5 13 15 Research and Development Plan– How will you execute on the Specific Aims? (Limited to 4 pages including text and graphics): Version 5 13 15 Proposed Product Pathway – What proposed product will result from this work? What is the regulatory path? How will a doctor and hospital approach value analysis for the product? (Limited to 2 pages including text and graphics.) Version 5 13 15 Commercialization – how will this award substantially enhance strength of an application for SBIR, STTR, or similar external funding? (Limited to 1 page including text and graphics.) Version 5 13 15 Description of the interdisciplinary nature of the project, the structure of the team, and plans for collaboration. (Limited to 2 pages including text and graphics.) Version 5 13 15 Selected references. As needed Version 5 13 15 Resources and environment. (Limited to 1 page including text and graphics.) Version 5 13 15 Other Support This section should include a full description of other support, including all current and pending sources of funds with amounts and dates for each for all sources (external and internal, including any start-up funds). The team leader and other faculty participants should list their other research support, highlighting any support that is available for the proposed project. Version 5 13 15 Detailed budget Detailed budget and justification in template word file. A budget section should be included with a justification of why the funds are necessary and how they will be used to promote innovative new science and secure applications for extramural funding. See RFA for funding limits. Version 5 13 15 Program Director/Principal Investigator (Last, First, Middle): FROM DETAILED BUDGET FOR INITIAL BUDGET PERIOD DIRECT COSTS ONLY THROUGH List PERSONNEL (Applicant organization only) Use Cal, Acad, or Summer to Enter Months Devoted to Project Enter Dollar Amounts Requested (omit cents) for Salary Requested and Fringe Benefits NAME ROLE ON PROJECT Cal. Mnths Acad. Mnths Summer Mnths INST.BASE SALARY SALARY REQUESTED FRINGE BENEFITS TOTAL PD/PI SUBTOTALS CONSULTANT COSTS EQUIPMENT (Itemize) SUPPLIES (Itemize by category) TRAVEL INPATIENT CARE COSTS OUTPATIENT CARE COSTS ALTERATIONS AND RENOVATIONS (Itemize by category) OTHER EXPENSES (Itemize by category) CONSORTIUM/CONTRACTUAL COSTS DIRECT COSTS TOTAL DIRECT COSTS FOR INITIAL BUDGET PERIOD PHS 398 (Rev. 6/09) $ Page Version 5 13 15 Form Page 4 Program Director/Principal Investigator (Last, First, Middle): FROM DETAILED BUDGET FOR INITIAL BUDGET PERIOD DIRECT COSTS ONLY THROUGH List PERSONNEL (Applicant organization only) Use Cal, Acad, or Summer to Enter Months Devoted to Project Enter Dollar Amounts Requested (omit cents) for Salary Requested and Fringe Benefits NAME ROLE ON PROJECT Cal. Mnths Acad. Mnths Summer Mnths INST.BASE SALARY SALARY REQUESTED FRINGE BENEFITS TOTAL PD/PI SUBTOTALS CONSULTANT COSTS EQUIPMENT (Itemize) SUPPLIES (Itemize by category) TRAVEL INPATIENT CARE COSTS OUTPATIENT CARE COSTS ALTERATIONS AND RENOVATIONS (Itemize by category) OTHER EXPENSES (Itemize by category) CONSORTIUM/CONTRACTUAL COSTS DIRECT COSTS TOTAL DIRECT COSTS FOR INITIAL BUDGET PERIOD PHS 398 (Rev. 6/09) $ Page Version 5 13 15 Form Page 4 Budget Justification A detailed budget justification is required for all costs. Please use additional pages if needed. Version 5 13 15