Chapter 11
Properties of
Solutions
Chapter 11
Table of Contents
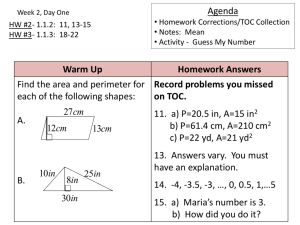
11.1
11.2
11.3
11.4
11.5
11.6
11.7
11.8
Solution Composition
The Energies of Solution Formation
Factors Affecting Solubility
The Vapor Pressures of Solutions
Boiling-Point Elevation and Freezing-Point
Depression
Osmotic Pressure
Colligative Properties of Electrolyte Solutions
Colloids
Copyright © Cengage Learning. All rights reserved
2
Section 11.1
Solution Composition
Various Types of Solutions
Example
State of
Solution
State of Solute
State of
Solvent
Air, natural gas
Gas
Gas
Gas
Vodka, antifreeze
Liquid
Liquid
Liquid
Brass
Solid
Solid
Solid
Carbonated water (soda)
Liquid
Gas
Liquid
Seawater, sugar solution
Liquid
Solid
Liquid
Hydrogen in platinum
Solid
Gas
Solid
Return to TOC
Copyright © Cengage Learning. All rights reserved
3
Section 11.1
Solution Composition
Solution Composition
moles of solute
Molarity (M ) =
liters of solution
mass of solute
Mass (weight) percent =
100%
mass of solution
moles A
Mole fraction ( A ) =
total moles of solution
moles of solute
Molality (m ) =
kilogram of solvent
Copyright © Cengage Learning. All rights reserved
Return to TOC
4
Section 11.1
Solution Composition
Molarity
moles of solute
Molarity (M ) =
liters of solution
Return to TOC
Copyright © Cengage Learning. All rights reserved
5
Section 11.1
Solution Composition
Exercise
You have 1.00 mol of sugar in 125.0 mL of
solution. Calculate the concentration in units
of molarity.
8.00 M
Return to TOC
Copyright © Cengage Learning. All rights reserved
6
Section 11.1
Solution Composition
Exercise
You have a 10.0 M sugar solution. What
volume of this solution do you need to have
2.00 mol of sugar?
0.200 L
Return to TOC
Copyright © Cengage Learning. All rights reserved
7
Section 11.1
Solution Composition
Exercise
Consider separate solutions of NaOH and KCl
made by dissolving 100.0 g of each solute in
250.0 mL of solution. Calculate the
concentration of each solution in units of
molarity.
10.0 M NaOH
5.37 M KCl
Return to TOC
Copyright © Cengage Learning. All rights reserved
8
Section 11.1
Solution Composition
Mass Percent
mass of solute
Mass (weight) percent =
100%
mass of solution
Return to TOC
Copyright © Cengage Learning. All rights reserved
9
Section 11.1
Solution Composition
Exercise
What is the percent-by-mass concentration of
glucose in a solution made my dissolving 5.5 g
of glucose in 78.2 g of water?
6.6%
Return to TOC
Copyright © Cengage Learning. All rights reserved
10
Section 11.1
Solution Composition
Mole Fraction
molesA
Mole fraction ( A ) =
total moles of solution
Return to TOC
Copyright © Cengage Learning. All rights reserved
11
Section 11.1
Solution Composition
Exercise
A solution of phosphoric acid was made by
dissolving 8.00 g of H3PO4 in 100.0 mL of
water. Calculate the mole fraction of H3PO4.
(Assume water has a density of 1.00 g/mL.)
0.0145
Return to TOC
Copyright © Cengage Learning. All rights reserved
12
Section 11.1
Solution Composition
Molality
moles of solute
Molality (m) =
kilogram of solvent
Return to TOC
Copyright © Cengage Learning. All rights reserved
13
Section 11.1
Solution Composition
Exercise
A solution of phosphoric acid was made by
dissolving 8.00 g of H3PO4 in 100.0 mL of
water. Calculate the molality of the solution.
(Assume water has a density of 1.00 g/mL.)
0.816 m
Return to TOC
Copyright © Cengage Learning. All rights reserved
14
Section 11.2
Atomic
The
Energies
Masses
of Solution Formation
Formation of a Liquid Solution
1. Separating the solute into its individual
components (expanding the solute).
2. Overcoming intermolecular forces in the solvent
to make room for the solute (expanding the
solvent).
3. Allowing the solute and solvent to interact to
form the solution.
Return to TOC
Copyright © Cengage Learning. All rights reserved
15
Section 11.2
Atomic
The
Energies
Masses
of Solution Formation
Steps in the Dissolving Process
Return to TOC
Copyright © Cengage Learning. All rights reserved
16
Section 11.2
Atomic
The
Energies
Masses
of Solution Formation
Steps in the Dissolving Process
• Steps 1 and 2 require energy, since forces must
be overcome to expand the solute and solvent.
• Step 3 usually releases energy.
• Steps 1 and 2 are endothermic, and step 3 is
often exothermic.
Return to TOC
Copyright © Cengage Learning. All rights reserved
17
Section 11.2
Atomic
The
Energies
Masses
of Solution Formation
Enthalpy (Heat) of Solution
• Enthalpy change associated with the formation
of the solution is the sum of the ΔH values for
the steps:
ΔHsoln = ΔH1 + ΔH2 + ΔH3
• ΔHsoln may have a positive sign (energy
absorbed) or a negative sign (energy released).
Return to TOC
Copyright © Cengage Learning. All rights reserved
18
Section 11.2
Atomic
The
Energies
Masses
of Solution Formation
Enthalpy (Heat) of Solution
Return to TOC
Copyright © Cengage Learning. All rights reserved
19
Section 11.2
Atomic
The
Energies
Masses
of Solution Formation
Concept Check
Explain why water and oil (a long chain
hydrocarbon) do not mix. In your explanation,
be sure to address how ΔH plays a role.
Return to TOC
Copyright © Cengage Learning. All rights reserved
20
Section 11.2
Atomic
The
Energies
Masses
of Solution Formation
The Energy Terms for Various Types of Solutes and Solvents
H1
H2
H3
Hsoln
Outcome
Polar solute, polar solvent
Large
Large
Large, negative
Small
Solution forms
Nonpolar solute, polar solvent
Small
Large
Small
Large, positive
No solution
forms
Nonpolar solute, nonpolar
solvent
Small
Small
Small
Small
Solution forms
Polar solute, nonpolar solvent
Large
Small
Small
Large, positive
No solution
forms
Return to TOC
Copyright © Cengage Learning. All rights reserved
21
Section 11.2
Atomic
The
Energies
Masses
of Solution Formation
In General
• One factor that favors a process is an increase
in probability of the state when the solute and
solvent are mixed.
• Processes that require large amounts of energy
tend not to occur.
• Overall, remember that “like dissolves like”.
Return to TOC
Copyright © Cengage Learning. All rights reserved
22
Section 11.3
The MoleAffecting Solubility
Factors
• Structural Effects:
Polarity
• Pressure Effects:
Henry’s law
• Temperature Effects:
Affecting aqueous solutions
Return to TOC
Copyright © Cengage Learning. All rights reserved
23
Section 11.3
The MoleAffecting Solubility
Factors
Pressure Effects
• Henry’s law:
C
k
P
=
=
=
C = kP
concentration of dissolved gas
constant
partial pressure of gas solute
above the solution
• Amount of gas dissolved in a solution is directly
proportional to the pressure of the gas above
the solution.
Return to TOC
Copyright © Cengage Learning. All rights reserved
24
Section 11.3
The MoleAffecting Solubility
Factors
A Gaseous Solute
Return to TOC
Copyright © Cengage Learning. All rights reserved
25
Section 11.3
The MoleAffecting Solubility
Factors
Temperature Effects (for Aqueous Solutions)
• Although the solubility of most solids in water
increases with temperature, the solubilities of
some substances decrease with increasing
temperature.
• Predicting temperature dependence of solubility
is very difficult.
• Solubility of a gas in solvent typically decreases
with increasing temperature.
Return to TOC
Copyright © Cengage Learning. All rights reserved
26
Section 11.3
The MoleAffecting Solubility
Factors
The Solubilities of
Several Solids as a
Function of
Temperature
Return to TOC
Copyright © Cengage Learning. All rights reserved
27
Section 11.3
The MoleAffecting Solubility
Factors
The Solubilities of
Several Gases in
Water
Return to TOC
Copyright © Cengage Learning. All rights reserved
28
Section 11.4
The Vapor Pressures of Solutions
An Aqueous Solution and Pure Water in a Closed Environment
Return to TOC
Copyright © Cengage Learning. All rights reserved
29
Section 11.4
The Vapor Pressures of Solutions
Liquid/Vapor Equilibrium
Return to TOC
Copyright © Cengage Learning. All rights reserved
30
Section 11.4
The Vapor Pressures of Solutions
Vapor Pressure Lowering: Addition of a Solute
Return to TOC
Copyright © Cengage Learning. All rights reserved
31
Section 11.4
The Vapor Pressures of Solutions
Vapor Pressures of Solutions
• Nonvolatile solute lowers the vapor pressure of
a solvent.
• Raoult’s Law: Psoln = solvPsolv
Psoln =
observed vapor pressure of solution
solv
Psolv
=
=
mole fraction of solvent
vapor pressure of pure solvent
Return to TOC
Copyright © Cengage Learning. All rights reserved
32
Section 11.4
The Vapor Pressures of Solutions
A Solution Obeying
Raoult’s Law
Return to TOC
Copyright © Cengage Learning. All rights reserved
33
Section 11.4
The Vapor Pressures of Solutions
Nonideal Solutions
• Liquid-liquid solutions where both components
are volatile.
• Modified Raoult’s Law:
PTotal = APA + BPB
• Nonideal solutions behave ideally as the mole
fractions approach 0 and 1.
Return to TOC
Copyright © Cengage Learning. All rights reserved
34
Section 11.4
The Vapor Pressures of Solutions
Vapor Pressure for a Solution of Two Volatile Liquids
Return to TOC
Copyright © Cengage Learning. All rights reserved
35
Section 11.4
The Vapor Pressures of Solutions
Summary of the Behavior of Various Types of Solutions
Interactive Forces Between
Solute (A) and Solvent (B)
Particles
Hsoln
T for Solution
Formation
Deviation
from
Raoult’s Law
Example
A A, B B A B
Zero
Zero
None (ideal
solution)
Benzenetoluene
A A, B B < A B
Negative
(exothermic)
Positive
Negative
Acetonewater
A A, B B > A B
Positive
(endothermic)
Negative
Positive
Ethanolhexane
Return to TOC
Copyright © Cengage Learning. All rights reserved
36
Section 11.4
The Vapor Pressures of Solutions
Concept Check
For each of the following solutions, would you
expect it to be relatively ideal (with respect to
Raoult’s Law), show a positive deviation, or
show a negative deviation?
a) Hexane (C6H14) and chloroform (CHCl3)
b) Ethyl alcohol (C2H5OH) and water
c) Hexane (C6H14) and octane (C8H18)
Return to TOC
Copyright © Cengage Learning. All rights reserved
37
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Colligative Properties
• Depend only on the number, not on the
identity, of the solute particles in an ideal
solution:
Boiling-point elevation
Freezing-point depression
Osmotic pressure
Return to TOC
Copyright © Cengage Learning. All rights reserved
38
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Boiling-Point Elevation
• Nonvolatile solute elevates the boiling point of
the solvent.
• ΔT = Kbmsolute
ΔT = boiling-point elevation
Kb
= molal boiling-point elevation constant
msolute= molality of solute
Return to TOC
Copyright © Cengage Learning. All rights reserved
39
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Boiling Point Elevation: Liquid/Vapor Equilibrium
Return to TOC
Copyright © Cengage Learning. All rights reserved
40
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Boiling Point Elevation: Addition of a Solute
Return to TOC
Copyright © Cengage Learning. All rights reserved
41
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Boiling Point Elevation: Solution/Vapor Equilibrium
Return to TOC
Copyright © Cengage Learning. All rights reserved
42
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Freezing-Point Depression
• When a solute is dissolved in a solvent, the
freezing point of the solution is lower than that
of the pure solvent.
• ΔT = Kfmsolute
ΔT = freezing-point depression
Kf
= molal freezing-point depression constant
msolute= molality of solute
Return to TOC
Copyright © Cengage Learning. All rights reserved
43
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Freezing Point Depression: Solid/Liquid Equilibrium
Return to TOC
Copyright © Cengage Learning. All rights reserved
44
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Freezing Point Depression: Addition of a Solute
Return to TOC
Copyright © Cengage Learning. All rights reserved
45
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Freezing Point Depression: Solid/Solution Equilibrium
Return to TOC
Copyright © Cengage Learning. All rights reserved
46
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Changes in Boiling Point and Freezing Point of Water
Return to TOC
Copyright © Cengage Learning. All rights reserved
47
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Exercise
A solution was prepared by dissolving 25.00 g
glucose in 200.0 g water. The molar mass of
glucose is 180.16 g/mol. What is the boiling
point of the resulting solution (in °C)? Glucose
is a molecular solid that is present as individual
molecules in solution.
100.35 °C
Return to TOC
Copyright © Cengage Learning. All rights reserved
48
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Exercise
You take 20.0 g of a sucrose (C12H22O11) and
NaCl mixture and dissolve it in 1.0 L of water.
The freezing point of this solution is found to
be -0.426°C. Assuming ideal behavior,
calculate the mass percent composition of the
original mixture, and the mole fraction of
sucrose in the original mixture.
72.8% sucrose and 27.2% sodium chloride;
mole fraction of the sucrose is 0.313
Copyright © Cengage Learning. All rights reserved
Return to TOC
49
Section 11.5
Boiling-Point Elevation and Freezing-Point Depression
Exercise
A plant cell has a natural concentration of
0.25 m. You immerse it in an aqueous solution
with a freezing point of –0.246°C. Will the
cell explode, shrivel, or do nothing?
Return to TOC
Copyright © Cengage Learning. All rights reserved
50
Section 11.6
Osmotic Pressure
• Osmosis – flow of solvent into the solution
through a semipermeable membrane.
• = MRT
M
R
T
=
=
=
=
osmotic pressure (atm)
molarity of the solution
gas law constant
temperature (Kelvin)
Return to TOC
Copyright © Cengage Learning. All rights reserved
51
Section 11.6
Osmotic Pressure
Return to TOC
Copyright © Cengage Learning. All rights reserved
52
Section 11.6
Osmotic Pressure
Osmosis
Return to TOC
Copyright © Cengage Learning. All rights reserved
53
Section 11.6
Osmotic Pressure
Return to TOC
Copyright © Cengage Learning. All rights reserved
54
Section 11.6
Osmotic Pressure
Exercise
When 33.4 mg of a compound is dissolved in
10.0 mL of water at 25°C, the solution has an
osmotic pressure of 558 torr. Calculate the
molar mass of this compound.
111 g/mol
Return to TOC
Copyright © Cengage Learning. All rights reserved
55
Section 11.7
Colligative Properties of Electrolyte Solutions
van’t Hoff Factor, i
• The relationship between the moles of
solute dissolved and the moles of particles
in solution is usually expressed as:
moles of particles in solution
i =
moles of solute dissolved
Return to TOC
Copyright © Cengage Learning. All rights reserved
56
Section 11.7
Colligative Properties of Electrolyte Solutions
Ion Pairing
• At a given instant a small percentage of the
sodium and chloride ions are paired and thus
count as a single particle.
Return to TOC
Copyright © Cengage Learning. All rights reserved
57
Section 11.7
Colligative Properties of Electrolyte Solutions
Examples
• The expected value for i can be determined for
a salt by noting the number of ions per formula
unit (assuming complete dissociation and that
ion pairing does not occur).
NaCl
KNO3
Na3PO4
i=2
i=2
i=4
Return to TOC
Copyright © Cengage Learning. All rights reserved
58
Section 11.7
Colligative Properties of Electrolyte Solutions
Ion Pairing
• Ion pairing is most important in
concentrated solutions.
• As the solution becomes more dilute, the
ions are farther apart and less ion pairing
occurs.
• Ion pairing occurs to some extent in all
electrolyte solutions.
• Ion pairing is most important for highly
charged ions.
Return to TOC
Copyright © Cengage Learning. All rights reserved
59
Section 11.7
Colligative Properties of Electrolyte Solutions
Modified Equations
T = imK
= iMRT
Return to TOC
Copyright © Cengage Learning. All rights reserved
60
Section 11.8
Colloids
• A suspension of tiny particles in some
medium.
• Tyndall effect – scattering of light by
particles.
• Suspended particles are single large
molecules or aggregates of molecules or
ions ranging in size from 1 to 1000 nm.
Return to TOC
Copyright © Cengage Learning. All rights reserved
61
Section 11.8
Colloids
Types of Colloids
Return to TOC
Copyright © Cengage Learning. All rights reserved
62
Section 11.8
Colloids
Coagulation
• Destruction of a colloid.
• Usually accomplished either by heating or
by adding an electrolyte.
Return to TOC
Copyright © Cengage Learning. All rights reserved
63